Scientific Achievement
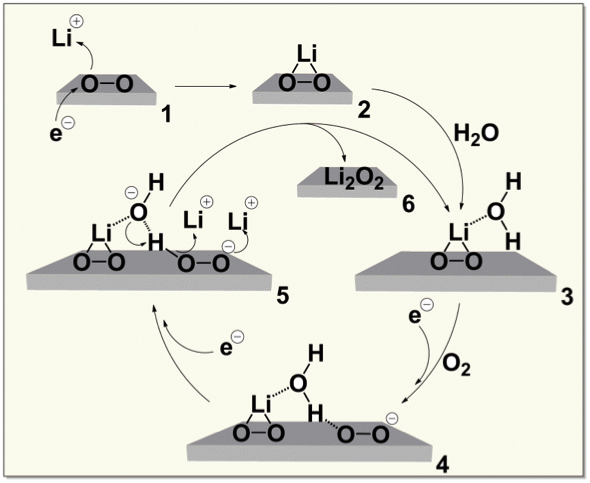
Water at ppm levels catalyzes the conversion of lithium superoxide (LiO2) to lithium peroxide (Li2O2) by the reaction cycle shown. Because water is not consumed in the cycle, trace amounts leverage large effects.
Significance and Impact
- Trace water controls the rate and outcome of the discharge reaction in lithium-air batteries.
- Reversing the lithium peroxide reaction, a primary challenge for lithium-air batteries, requires understanding the role of trace water, an unexplored area.
- The strong polarity and active electrochemistry of trace water make it a likely player in many battery phenomena including solvation, the double layer, and redox behavior, all uncharted territory.
Research Details
- Novel procedure for elimination of water to <1 ppm from organic solvents, followed by controlled dilution
- The wet Electrochemical Discovery Laboratory (EDL) with exceptional purification and multi-modal Raman, FTIR, RRDE and AFM characterization enabled this research.
Work performed at Argonne National Laboratory, Sandia National Laboratory, University of Illinois at Urbana-Champaign and Northwestern University Performers: Jakub Jirkovsky, Ram Subbaraman, Dusan Strmcnik, Katharine Harrison, Charles Diesendruck, Rajeev Assary, Otakar Frank, Lukas Kobr, Gustav Wiberg, Bostjan Genorio, Vojislav Stamenkovic, Larry Curtiss, Jeffrey Moore, Kevin Zavadil, and Nenad Markovic

