Scientific Achievement
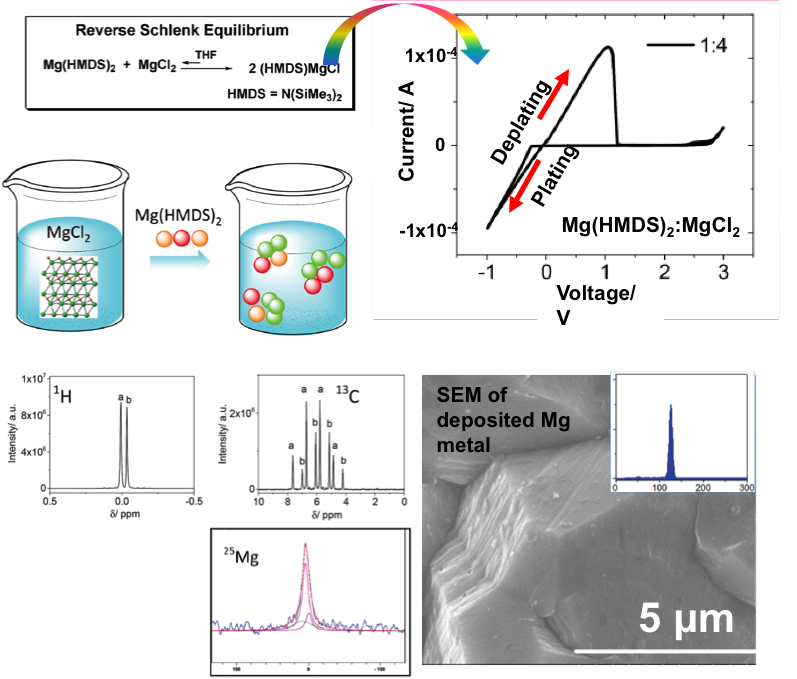
A simple mixture of magnesium compounds: magnesium hexamethyldisilazide (Mg(HMDS)2) and magnesium chloride (MgCl2) was prepared to achieve reversible Mg deposition/dissolution, a wide electrochemical window, and a coulombic efficiency of 99%.
Significance and Impact
The unexpected high solubility of MgCl2 in the solvent of THF with the help from Mg(HMDS)2 provides a new way to develop magnesium electrolytes.
Research Details
In sum, the reverse Schlenk equilibrium mechanism (Scheme 1) resulted in the development of a novel magnesium electrolyte (Mg(HMDS)2/MgCl2) with improved coulombic efficiency (>99%) and electrochemical window (2.8 V vs Mg2+/Mg). The success key of the invention of this Mg(HMDS)2/MgCl2 is the superior solubility of MgCl2 in THF.
Work performed at Argonne National Laboratory (JCESR managing partner) by C. Liao, N. Sa, B. Key, A. K Burrell, L. Cheng, L. A Curtiss, J. T Vaughey, J-J Woo, L. Hu, B. Pan and Z. Zhang, J. Mater. Chem. A., 2015.
DOI: 10.1039/C5TA00118H

