Scientific Achievement
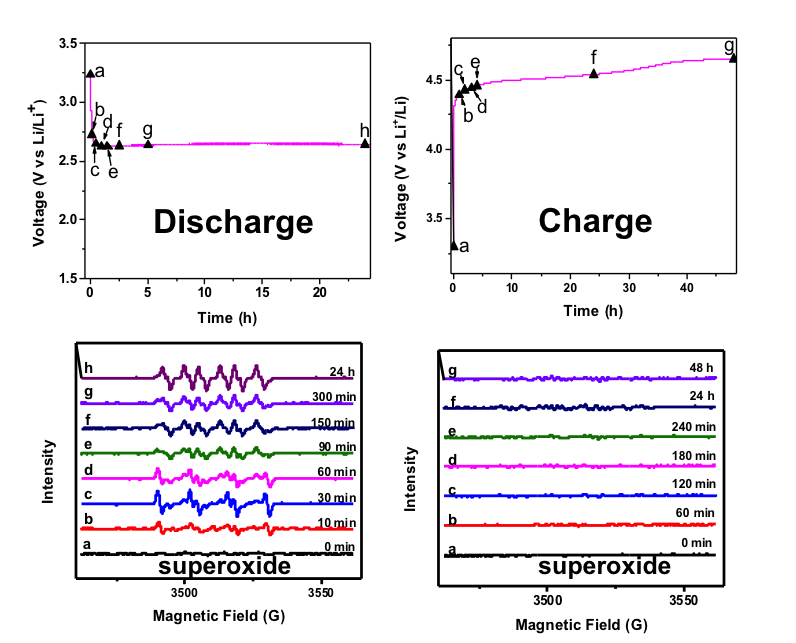
Superoxide radical anions (O2•−) are intermediates formed in non-aqueous lithium-oxygen batteries, which are very reactive but have a very short lifetime. Using 5,5-dimethyl-pyrroline N-oxide as a spin trap, we stabilized these radicals and enabled direct verification of superoxide radicals by electron paramagnetic resonance (EPR).
Significance and Impact
A fundamental understanding of the mechanisms of both the oxygen reduction reaction and the oxygen evolution reaction in non-aqueous lithium-oxygen batteries is essential for the further development of these batteries.
Research Details
- EPR results show O2•− is generated in the oxygen reduction reaction during the discharge process of a Li-O2 battery when using a DMSO-based electrolyte, while no signal corresponding to O2•− is detected in the oxygen evolution reaction during the charge process of a Li-O2 battery.
- The chemical compositions of the discharge products were further analyzed.
Work performed at Pacific Northwest National Laboratory (JCESR partner), Environmental Molecular Sciences Laboratory, by R. Cao, E. Walter, W. Xu, E. Nasybulin, P. Bhattacharya, M. Bowden, M. Engelhard and J. G. Zhang. ChemSusChem, 2014, 7, 2436-2440.

