Scientific Achievement
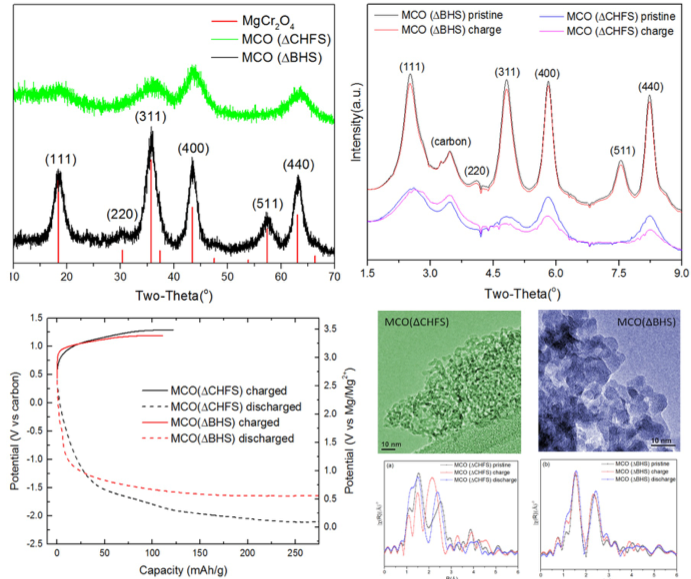
Reversible magnesium removal from MgCr2O4 was induced by nanosizing and introducing significant structural defects to reduce diffusion the distance and overcome the activation energy barrier.
Significance and Impact
Tiny, disordered particles of magnesium chromium oxide exemplify the design and synthesis of new magnesium cathode materials with structural defects, enrich the understanding of Mg2+ intercalation reactions in oxides, and validate the divalent battery strategies with better energy storage than conventional lithium-ion batteries.
Research Details
- Ordered 7 nm crystals of spinel-type MgCr2O4 were synthesized by a conventional batch hydrothermal synthesis (ΔBHS) method. In comparison, the relatively underexplored Continuous Hydrothermal Flow Synthesis (ΔCHFS) method was used to make highly defective sub-5 nm MgCr2O4 crystals.
- The results of XRD, XANES, EXAFS and EDX analysis on charged and discharged electrodes of MCO(ΔCHFS) were consistent with bulk Mg2+ removal and insertion, with accompanying Cr redox activity. In contrast, there was no evidence of this in MCO(Δ BHS), which seemingly only displayed decomposition reactions, likely in concert with the electrolyte at the surface.

