Scientific Achievement
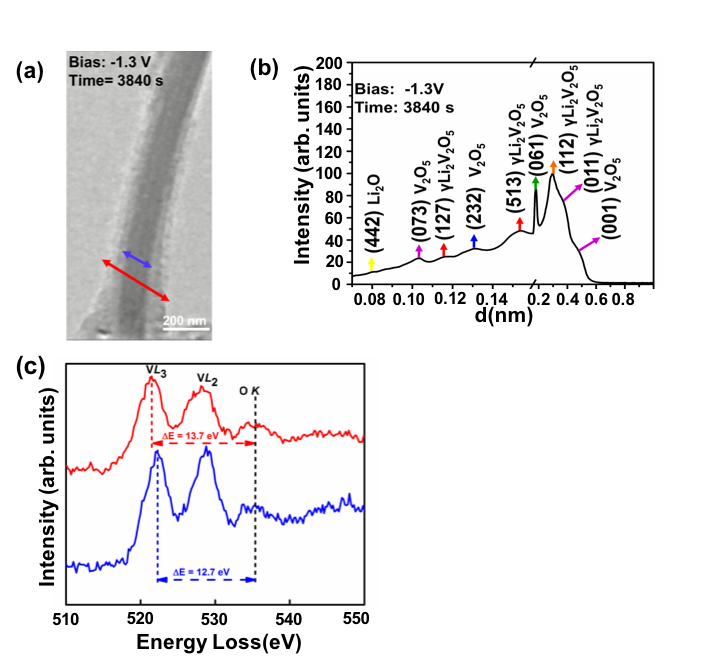
We examine the effects of Li intercalation into α-V2O5 nanowires using in situ transmission electron microscopy. Combining electron diffraction and electron energy loss spectroscopy, we conclude that the pristine V2O5 nanowires form a Li2O shell, which acts as a solid state electrolyte. Thus Li+ ions move radially into the nanowire core, and bulk of the nanowire undergoes transformation to the γ-Li2V2O5 phase at -1.3 V bias.
Significance and Impact
We demonstrate the structural evolution of α-V2O5 nanowire upon in situ lithiation. Our diffraction data identifies the formation of the γ-Li2V2O5 phase, which is further confirmed by EELS analysis. The V L-edge fine-structure reveals a valence state change from V5+ (pristine) to V4+(γ-Li2V2O5 ). A similar setup can be employed with an appropriate ionic liquid electrolyte (that can sustain high column vacuum inside TEM) to study Mg2+ intercalation into oxide based cathodes.
Research Details
- In situ lithiation experiments were done using the Nanofactory biasing holder, in an open cell battery setup.
Work performed at University of Illinois at Chicago (JCESR partner) by A. Mukherjee, H.A. Ardakani, T.Yi, J. Cabana, R.S Yassar, R.F Klie, Appl. Phys. Lett. 2017, 110, 213903

