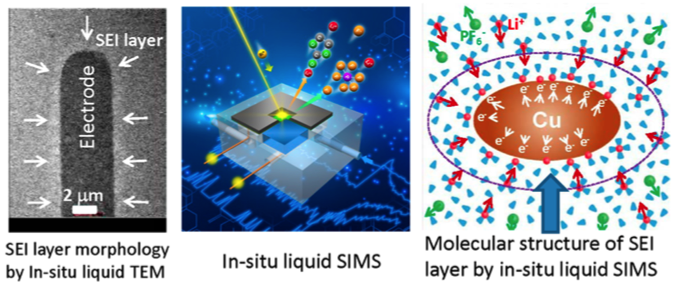
Direct probing the molecular structure of solid electrolyte interface layer operando for lithium-ion battery
Combining of in-situ TEM and in-situ liquid SIMS can yield both chemistry, structure and morphological information at solid-liquid interface
Scientific Achievement
Deposition of Li metal on copper electrode leads to condensation of solvent molecules around the electrode. Chemically, this layer of solvent condensate tends to be depleted of the salt anions and have reduced concentration of Li+ ions, essentially leading to formation of a lean electrolyte layer adjacent to the electrode and therefore contributing to the cell overpotential.
Significance and Impact
This observation provides unprecedented molecular level dynamic information on the initial formation of the solid electrolyte interphase (SEI) layer.
Research Details
- Creation and usage of in situ liquid secondary ion mass spectroscopy (SIMS) for the first time to directly observe the molecular structural evolution at the solid−liquid electrolyte interface for a lithium (Li)-ion battery under dynamic operating conditions
Work performed at Pacific Northwest National Laboratory (JCESR Partner) by Zihua Zhu, Yufan Zhou, Pengfei Yan, Rama Sesha Vemuri, Wu Xu, Rui Zhao, Xuelin Wang, Suntharampillai Thevuthasan, Donald R. Baer, and Chong-Min Wang, Nano Lett., 2015, 15, 6170-6176

