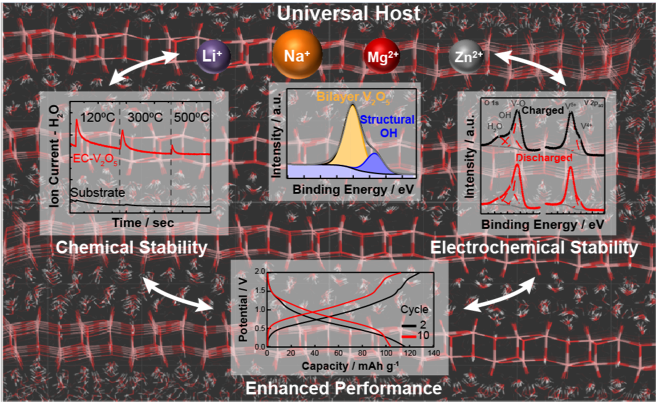
Scientific Achievement
Structural OH species, an intrinsic feature of electrochemically-synthesized V2O5 (EC-V2O5), are highly thermally and electrochemically stable and are responsible for the improved cycle life and reversible capacity of these materials for beyond-lithium-ion systems.
Significance and Impact
These insights have broad implications for understanding the performance of a variety of hydrated oxide systems, and indicate that the formation of covalently-bound OH species can lead to further improvements in the performance of nanoscale materials and ultimately enable the design of optimal structures that can operate as universal hosts with high capacity and dramatically improved cycle life.
Research Details
- Fourier Transform Infrared and Nuclear Magnetic Resonance Spectroscopy results clearly point to the presence of distinct proton/hydroxyl moieties in EC-V2O5, while Atom Probe Tomography analysis reveals their highly isotropic distribution, with no evidence of surface segregation
- In situ Ambient Pressure X-ray Photoelectron Spectroscopy (AP-XPS) results support Mass Spectrometry (MS) findings that the release of hydroxyl protons from structural OH groups takes place above 5000C, providing a clear map of the temperature-dependent speciation of hydrated EC-V2O5.

