![]() While today’s batteries use liquid electrolytes, solid electrolytes offer the promise of greater safety and higher performance through incorporation of advanced chemistries. Designing solid electrolytes for next-generation batteries requires deep knowledge of their atomic and molecular interactions with the working ion (Li+ in the case of lithium-ion batteries) and with each other, with special attention to the fundamental differences between liquids and solids.
While today’s batteries use liquid electrolytes, solid electrolytes offer the promise of greater safety and higher performance through incorporation of advanced chemistries. Designing solid electrolytes for next-generation batteries requires deep knowledge of their atomic and molecular interactions with the working ion (Li+ in the case of lithium-ion batteries) and with each other, with special attention to the fundamental differences between liquids and solids.
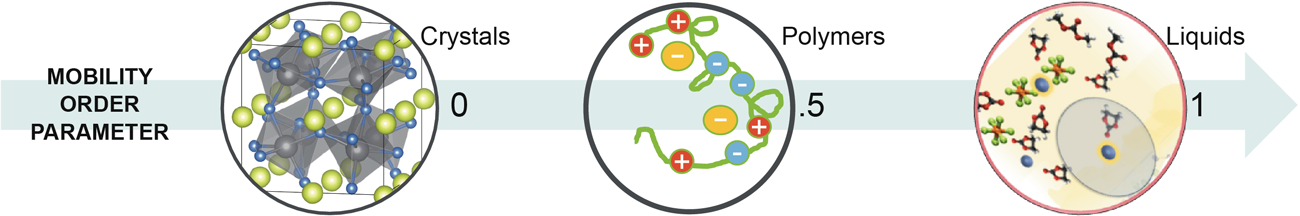
In liquids, the working ion dissolves by acquiring a solvation shell of surrounding solvent ions and molecules that moves with the ion as it diffuses through the liquid. In solids, the working ion resides in special positions within the solid determined by the interactions of the working ion with the fixed ions and molecules of the host solid. The fixed ions and molecules of the host solid form a rigid solvation “cage” around the working ion that traps the ion in its special position. In order to move, the working ion must hop from fixed solvation cage to fixed solvation cage, unlike in liquids where the working ion drags its solvation shell with it as it moves continuously through the liquid.
The Solid Solvation Thrust aims to develop the solvation cage description for all solid electrolytes. The Thrust has two objectives: developing the solvation cage description for soft pliable cages such as membranes and polymers, and for hard, brittle cages such as glasses and crystals. Its research will draw heavily on the crystalline simulation techniques developed using the Materials Project in JCESR’s first five years, and will incorporate extensive in situ X-ray, NMR and transport studies. The concept of fixed solvation cages in solids allows rich comparisons with the moveable solvation shells in liquids. Seen in this way, liquid electrolytes are the ultimate endpoint of soft pliable cages in solid electrolytes.