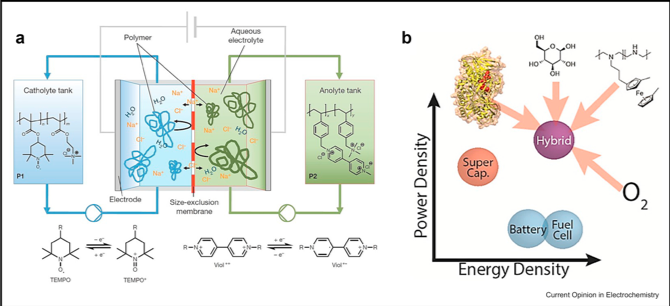
Scientific Achievement
Redox polymers were designed for mediation in sensors in the 1980s, but it was not until the last five years that researchers realized that they could play a critical role in energy storage, including redox flow batteries and supercapacitors.
Significance and Impact
This review details the historical progression of redox polymers from glucose sensors to energy storage, including discussion of the advances over the last decade in rationally designing redox polymers for the application of interest.
Research Details
- Background on the history of redox polymers for sensing applications
- History of the transition of redox polymers from sensors to energy conversion
- History of the transition of redox polymers to energy storage
- Discussion of the different rational design strategies depending on the application (i.e. redox flow battery versus fuel cell versus supercapacitor)

