Scientific Achievement
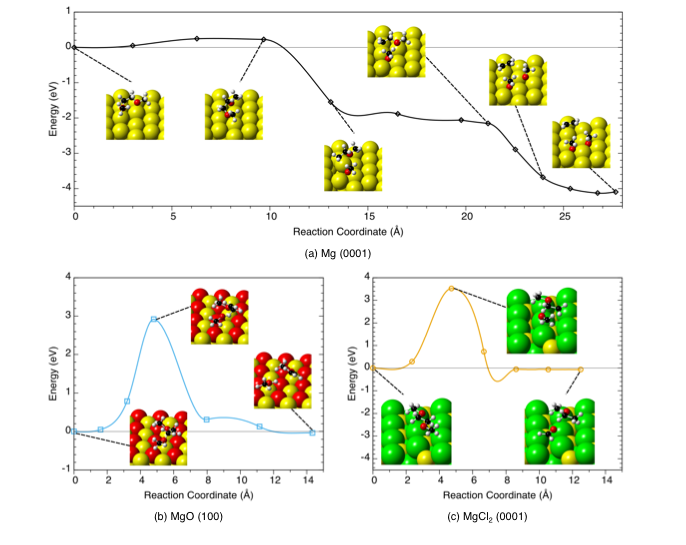
Dimethoxyethane (DME) is predicted to rapidly decompose to ethylene gas and other products on the metallic Mg surface, whereas the presence of an oxide or chloride surface film on a Mg anode is predicted to limit solvent decomposition.
Significance and Impact
The commercialization of Mg-ion batteries can be accelerated by tailoring the properties of the Mg anode surface film to limit solvent decomposition, yet allow for the rapid transport of Mg2+ across its thickness.
Research Details
- DFT calculations were performed to predict both the thermodynamic driving force (reaction enthalpy) and kinetics (activation energy) of plausible decomposition reactions on three Mg anode surface phases: Mg metal, MgO, and MgCl2.
- Using Bader Charge analysis, reductive charge transfer from the metallic electrode was shown to minimize reaction barriers and stabilize decomposition products.

