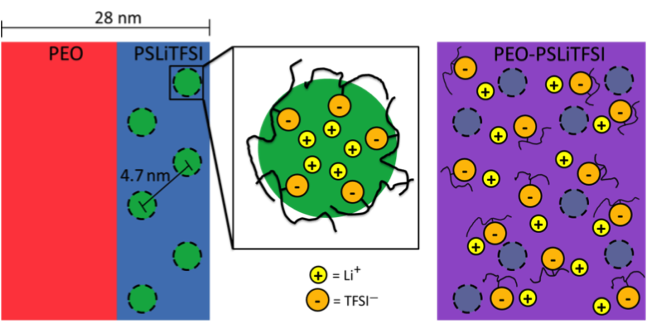
(Right) At high temperatures, block mixing occurs and lithium ions are released from clusters.
Scientific Achievement
Showed that an order to disorder transition in PEO-PSLiTFSI is qualitatively different than that in typical block copolymers. At this transition, the ionic conductivity increased several orders of magnitude.
Significance and Impact
Identified the relationship between ionic conductivity and morphology in a new class of single-ion-conducting block copolymer electrolyte. Such electrolytes may be ideal for lithium metal batteries.
Research Details
- Small angle X-ray scattering (SAXS) and transmission electron microscopy measurements show that PEO-PSLiTFSI has a long-range ordered lamellar morphology at low temperatures.
- An ordered to disordered (block mixing) transition was observed at the melting temperature with SAXS, when ionic conductivity increased significantly.
Work was performed at Lawrence Berkeley National Laboratory: the Molecular Foundry and the Advanced Light Source (beamline 7.3.3) by Inceoglu, S.; Rojas, A. A.; Devaux, D.; Chen, X. C.; Stone, G. M.; Balsara, N. P. Morphology–Conductivity Relationship Of Single-Ion-Conducting Block Copolymer Electrolytes for Lithium Batteries. ACS Macro Lett. 2014, 3, 510–514.
DOI: 10.1021/mz5001948

