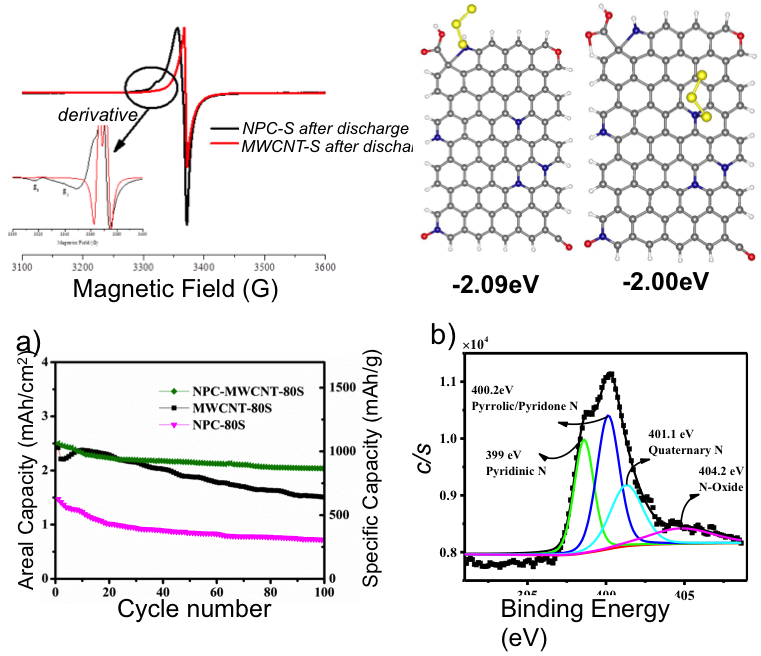
(Bottom) A high areal capacity of ca. 2.5 mAh/cm2 with capacity retention of 81.6% over 100 cycles.
Scientific Achievement
N-doped carbon stabilizes the S3 radicals, which are rarely detected in ether-based electrolyte due to their high reactivity; this also restrains the polysulfide loss during battery cycling.
Significance and Impact
The approach of using nitrogen-doped carbon to stabilize S3 radicals within the cathode scaffold presents a new mechanism to improve Li-S battery performance.
Research Details
- EPR and DFT results show S3 radicals become chemically stable toward the adsorption on the N-doped carbon, the total amount of the radicals increased compared to the pure CNT carbon surface.
- The combination of good conductive CNTs and N-doping functional groups of porous carbon, the uniform coating of thick sulfur electrode (2.5mg/cm2 S loading) and 81.6% capacity retention after 100 cycles are obtained.
Work performed at Pacific Northwest National Laboratory (JCESR partner) by Chen, J., Wu, D., Walter, E., Engelhard, M., Bhattacharya, P., Pan, H., Shao, Y., Gao, F., Xiao, J. and Liu, J. Nano Energy (2015) 13, 267–274.

