Scientific Achievement
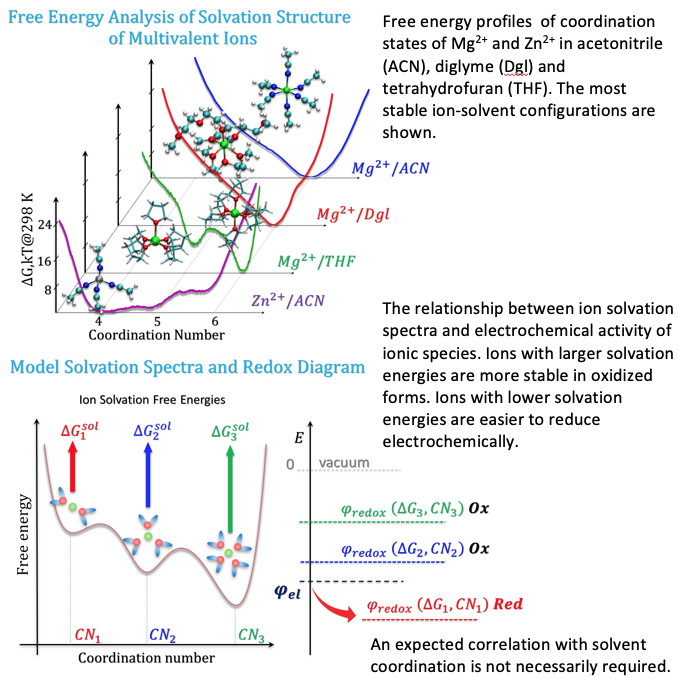
Advanced free-energy sampling techniques reveal that monoatomic divalent cations in organic solvents may have multiple well-defined minima or plateaus of free energy with respect to the number of coordinating solvent molecules. We introduce the concept of “ion solvation spectra” to highlight non-trivial phenomenology related to a continuum of ion solvation environments, as opposed to a single coordination minimum.
Significance and Impact
The multiplicity of stable ion-solvent coordination states implies a multiplicity of: (1) solvation free energies, (2) bulk redox potentials, and (3) ion transport properties, for a given cation and solvent. The least stable ion coordination states (the minor population) should be most electrochemically active (first to be reduced), and their presence may significantly alter the properties of electrochemical interfaces. This multiplicity of ion-solvent coordination environments defines an important microscale descriptor of electrolytes.
Research Details
- The results are based on both classical and ab initio molecular dynamics free energy sampling.
- We estimate kinetic barriers associated with the ion (de-)solvation processes.
- We highlight deficiencies of conventional methodologies which assume single, well-defined minima for ion solvation.

