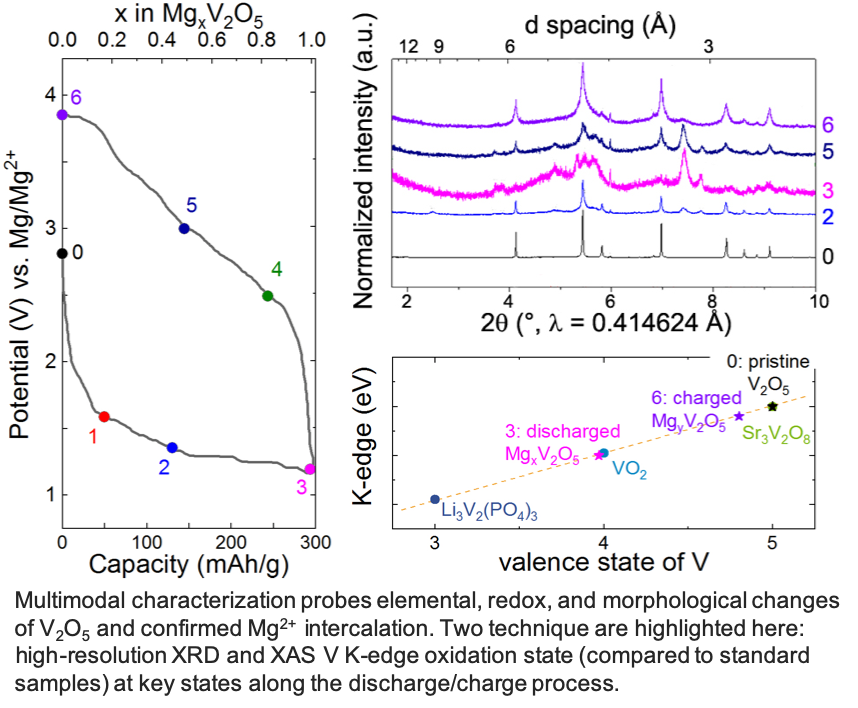
Scientific Achievement
Layered a-V2O5 reversibly intercalates 1 Mg2+ per unit formula to achieve 280 mAh/g at 110°C in a chemically and anodically stable ionic liquid electrolyte.
Significance and Impact
We find that our previous observations of materials complexity driven by the need of H2O participation can be decoupled from direct, solvent-free Mg interaction into V2O5. Formation of defects/microstructural formatting mitigate the electrochemical hysteresis on subsequent cycles. Through establishing control and understanding of these defects and transformation, this system can ultimately surpass the energy density of current lithium-ion batteries.
Research Details
- Complete materials characterization establishing conclusive proof of reversible Mg2+ intercalation in α-V2O5 without H+ or H2O co-insertion.
- Ionic liquid electrolytes at elevated temperatures mitigates hydration, kinetic limitations, and unintended side-reactions.
- Massive changes in microstructure, possibly driven by extensive defects upon magnesiation, subsequently support room temperature cycling.

