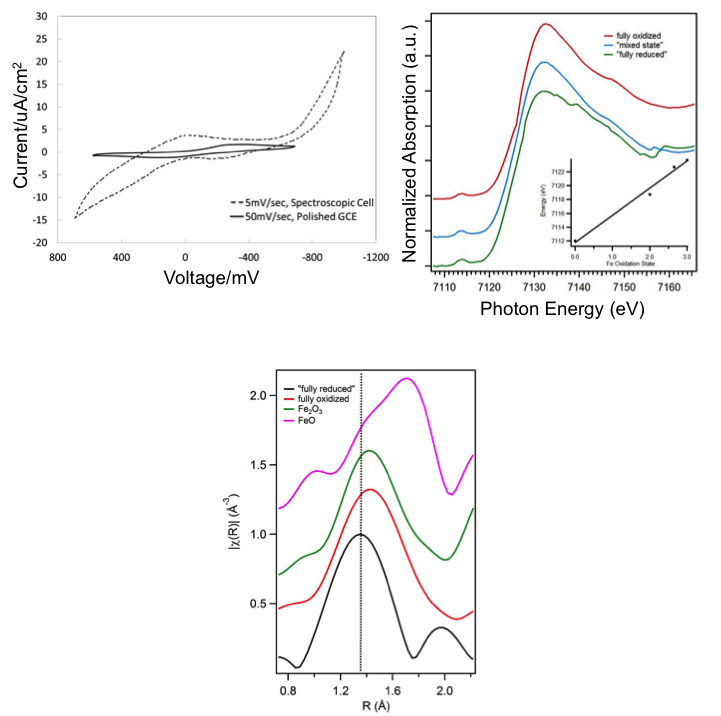
(Top Right) XANES spectra showing IL in fully oxidized and fully reduced states, showing change in Fe Kα edge on oxidation state change
(Bottom) EXAFS data showing position of fully oxidized (Fe+3) state of IL, vs. reduced state (Fe+2), showing inward motion of O neighbors, instead of the expected outward motion predicted from Fe2O3 structure
Scientific Achievement
Redox active ionic liquids have been studied using in situ electrochemical and spectroscopic techniques to demonstrate conformational change in ligand structure during redox behavior of the iron center.
Significance and Impact
Direct observation of the role of ligand binding around the redox site of a redox active ionic liquid indicates that the ligands play a role in the redox process, as the oxygen to iron spacing is not following the behavior of standard electrostatics on oxidation and reduction.
Research Details
- Electrochemical oxidation and reduction of redox active iron center ionic liquid in situ during XANES and EXAFS observations
- XANES indicates proper oxidation state change of the iron center commensurate with the cyclic voltammagrams taken on the IL, but only partial reduction, likely due to transport limitations
- EXAFS data indicate the Fe-O bond distance increases with Fe oxidation, which is contrary to electrostatics prediction, indicating a more complex role for the ligand structure during redox behavior
Work performed at Sandia National Laboratory (JCESR partner), University of Maine and Brookhaven National Laboratory by Apblett, C. A., Stewart, D. M., Fryer, R. T., Sell, J. C., Pratt III, H. D., Anderson, T. M., Meulenberg, R. W., Electrochim. Acta, 185 (2015) 156-161.

