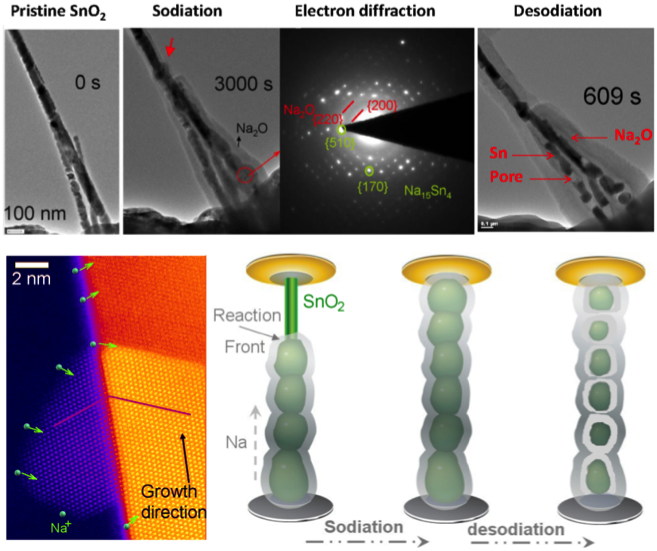
Scientific Achievement
Direct measurement reveals that Na ion diffusion is 30 times slower than Li ion. Na insertion softens nanowire and causes significant deformation to lead to easy deformation of the product. Pore formation upon Na ion removal leads to poor cycliability of the SnO2 electrode
Significance and Impact
- Understand large cation insertion mechanism beyond lithium ions
- Na diffuses much slower than Li in SnO2, direct correlation with charge/discharge rate of battery
- Identified the critical role of ionic size and electronic structure in searching for new charge carry ions
Research Details
- Combination of in-situ TEM and DFT calculation to probe into the Na ion transport and deformation behavior of SnO2 anode for sodium ion battery.
- Measured the Na ion transport rate in SnO2 and deformation behavior of the materials
- Compared the results with the case of Li
Meng Gu et al. Nano Letters, 2013, submitted. Meng Gu, Akihiro Kushima, Yuyan Shao, Ji-Guang Zhang, Jun Liu, Nigel D. Browning, Ju Li, and Chongmin Wang. PNNL and MIT

