Scientific Achievement
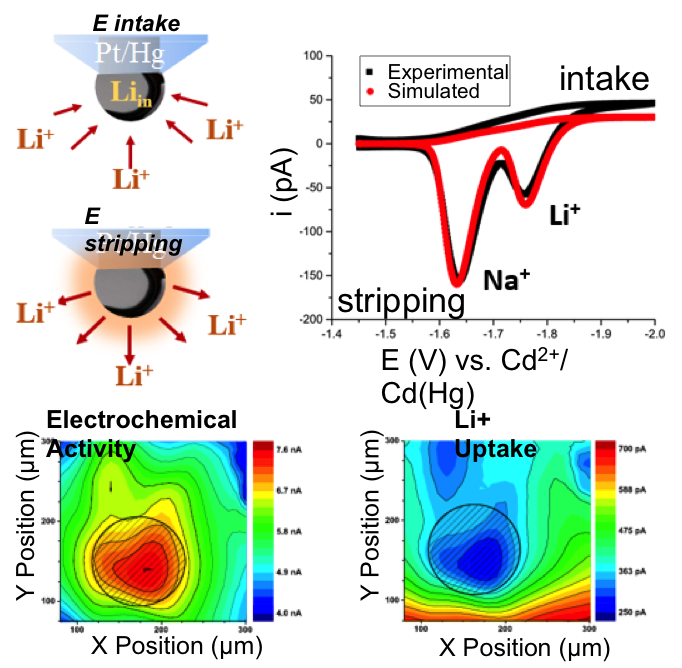
- Quantitative electrochemical probes for the in situ detection of reactive fluxes of alkaline ions in non-aqueous media were developed
- Probes can be used in direct (intake) and pre-concentration mode (stripping) for imaging the flux of ions from an electrochemically activated surface
- Implementation of technique onto a 120 nm nano-Hg electrode shows promising for battery nano-imaging
Significance and Impact
- Interfacial dynamics of ions in battery electrodes and membranes are poorly understood- imaging technique will target quantitatively heterogeneity in materials activity
- In situ imaging for evolving interfaces: SEI formation, dissolution/electrodeposition
- Currently in development for nano-imaging and use with multivalent species such as Mg2+and Ca2+
Research Details
- Li+, Na+ and K+ quantification in the micromolar to millimolar range using micro- and nano- Hg deposits on scanning electrochemical microscopy tips in propylene carbonate
- Proof of concept application to an Au substrate showing the effects of under-potential deposition on the local flux of Li+
Work performed at the University of Illinois at Urbana-Champaign (JCESR partner) by Barton, Z.J. and Rodriguez-Lopez, J., Anal. Chem., 2014.
DOI: 10.1021/ac502517b

