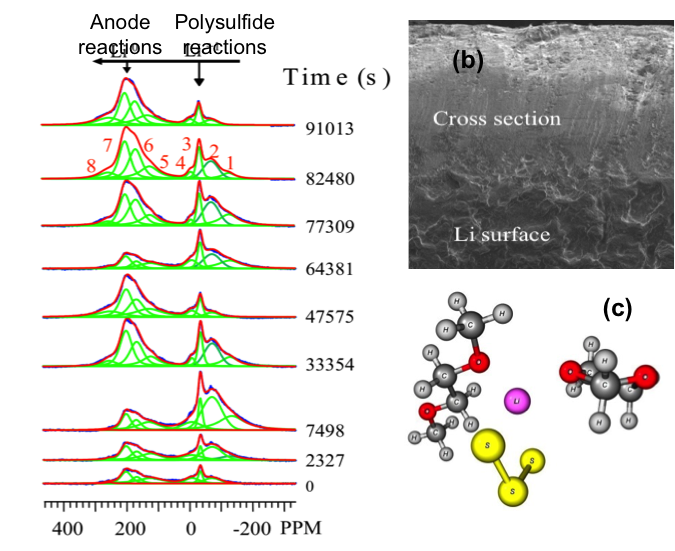
(b) Formation of a thick SEI layer on Li anodes causes the degradation of the battery.
(c) DFT calculation of the interaction of S3˙- radical (yellow) with polysulfide and electrolyte molecules.
Scientific Achievement
- First in situ NMR characterization of the full cell electrochemical reactions in Li-S batteries using a microbattery design
- Interphase (SEI) layer formation, evolution of anode surface roughness and dendrite nucleation – key features defining the performance and failure modes of next generation lithium batteries
Significance and Impact
- The environments of the polysulfides are very different during charge and discharge and the information can only be revealed by in situ experiments
- The study revealed transient free radicals responsible for battery performance degradation
Research Details
- A Li-S microbattery is designed in situ NMR experiments
- Redox reactions on the cathode, anode and in the electrolytes are tracked and quantified in real time
- Reactions relation to the formation and evolution of all polysulfide species are identified and quantified as a function of depth of charge and discharge
- The effect of free radicals on polysulfides is calculated using DFT methods
Worked performed at Pacific Northwest National Laboratory (JCESR partner) by Jie Xiao, Jian Zhi Hu, Honghao Chen, M. Vijayakumar, Jianming Zheng, Huilin Pan, Eric D. Walter, Mary Hu, Xuchu Deng, Ju Feng, Bor Yann Liaw, Meng Gu, Zhiqun Daniel Deng, Dongping Lv, Suochang Xu, Chongmin Wang and Jun Liu, Nano Letters, 2015.

