Scientific Achievement
Van der Waals-augmented density functional theory (vdW-DF), quasi-particle methods (G0W0), and continuum solvation techniques were used to predict several structural, thermodynamic, spectroscopic, electronic, and surface characteristics of solid-phase redox end-members in Li-S batteries.
Significance and Impact
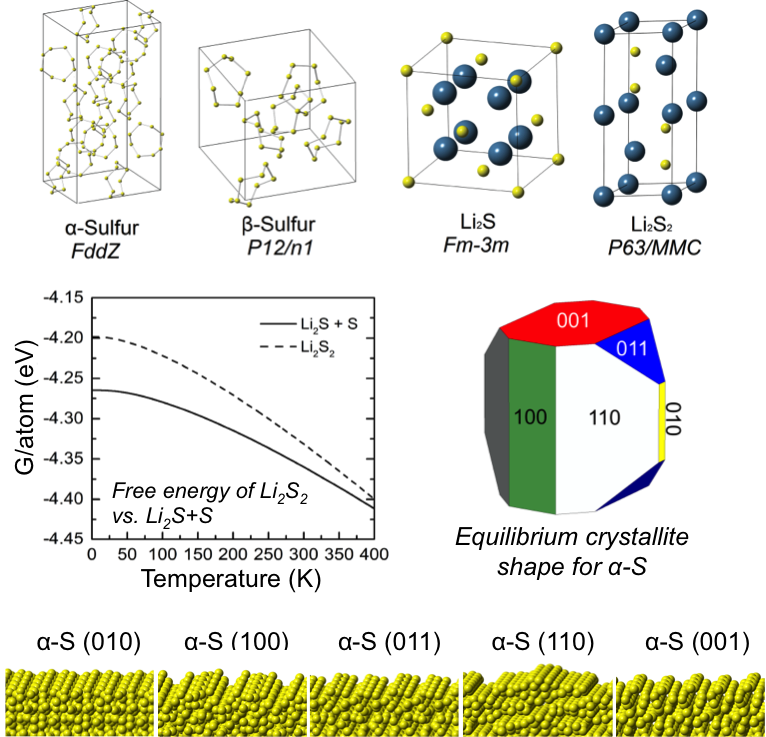
- The stability of lithium persulfide, Li2S2, a compound whose presence may limit the capacity of Li-S batteries, was assessed by comparing the energies of hypothetical A2B2 crystal structures.
- In all cases Li2S2 is predicted to be unstable with respect to a two-phase mixture of Li2S and α-S, suggesting that Li2S2 is a metastable phase.
Research Details
The stable surfaces and equilibrium crystallite shapes of Li2S and α-S were predicted in the presence of a continuum solvation field intended to mimic the effect of a dimethoxyethane (DME)-based electrolyte.
Work performed at the University of Michigan (JCESR partner) by H. Park, H. S. Koh and D. J Siegel. J. Phys. Chem. C., 2015, 119, 4675-4683.
DOI: 10.1021/jp513023v

