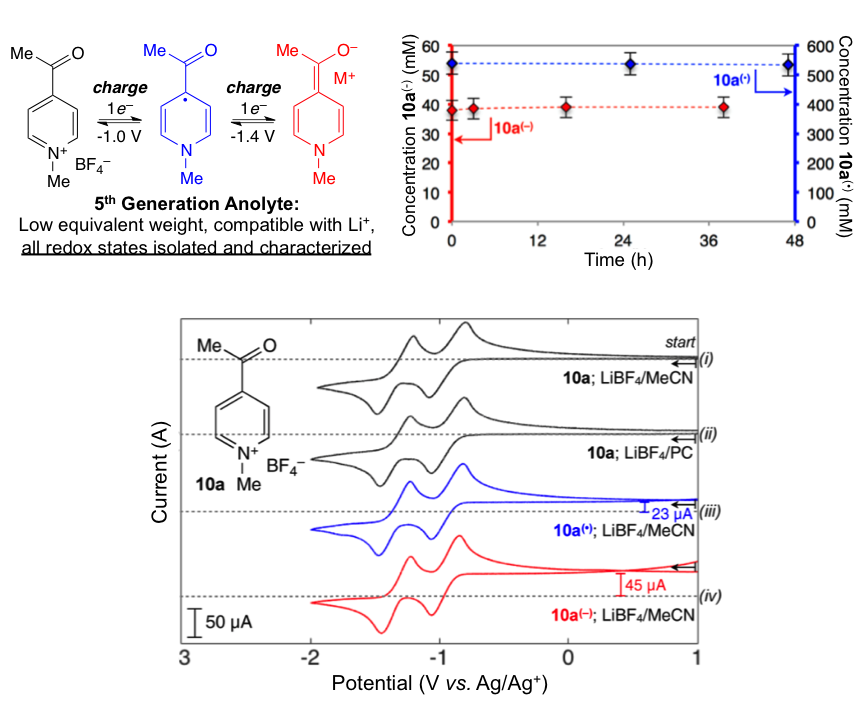
Scientific Achievement
All-organic anolyte materials for nonaqueous redox flow batteries with high stability at all redox states were designed through an iterative study. Anolyte materials exhibit two chemically reversible reductions, have low equivalent weights of only 111 g/mol/e–, undergo reversible electro-chemistry in Li-ion supporting electrolytes, and are stable in solution for greater than 2 months without detectable decomposition.
Significance and Impact
This study demonstrates a rare example of a nonaqueous organic anolyte material with high stability in Li-ion electrolytes that can be coupled against the many known organic catholyte materials to form an all-organic nonaqueous redox flow battery.
Research Details
- Trends in chemical reversibility were assessed for a large number of pyridines by CV.
- Chemical synthesis and isolation of charged materials was performed for more rigorous stability evaluation.
Work was performed at the University of Michigan (JCESR partner), University of Illinois at Urbana Champaign (JCESR partner), and Argonne National Laboratory (JCESR managing partner) by C. S. Sevov, R. E. M. Brooner, E. Chenard, R. S. Assary, J. S. Moore, J. Rodriguez-Lopez and M. S. Sanford, Journal of the American Chemical Society, 2015.

