Scientific Achievement
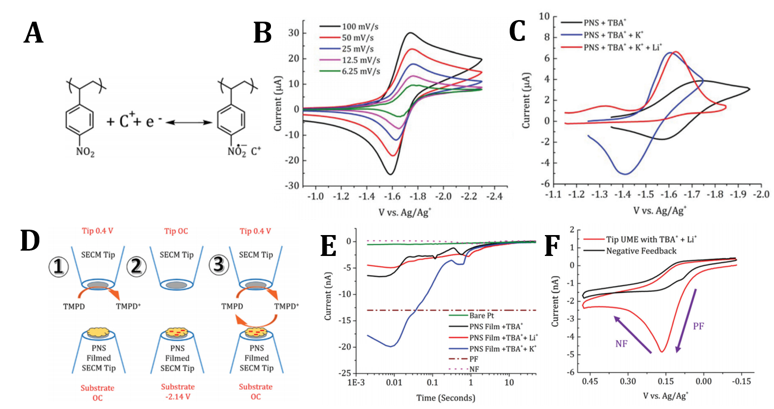
A redox active polymer (RAP) with nitro– substituents was synthesized and studied via voltammetric analysis in combination with surface interrogation and ionic sensitive scanning electrochemical microscopy to reveal specific electrolyte interactions on the performance of the material.
Significance and Impact
This study demonstrates the potential to improve the performance of an energy storage material via supporting electrolyte tuning. Utilizing low-weight redox active materials and identifying conditions for optimum reactivity could lead to flow batteries with a high specific energy density and higher accessible current densities.
Research Details
- Poly (para-nitrostyrene) (PNS) with a molecular weight of Mn 270 kDa was synthesized following a nitration of a polystyrene backbone.
- PNS was evaluated electrochemically in dimethylformamide (DMF) as solvent as PNS is highly soluble in DMF (~4 mol/L).
- PNS shows enhancement of faradaic currents by nearly 100% in the presence of K+ supporting electrolytes due to increased pendant accessibility.
- In K+ electrolytes PNS has at least a 40 fold improvement in the electrochemical kinetics compared to TBA+ and Li+ salts as evaluated by scanning electrochemical microscopy.

