Scientific Achievement
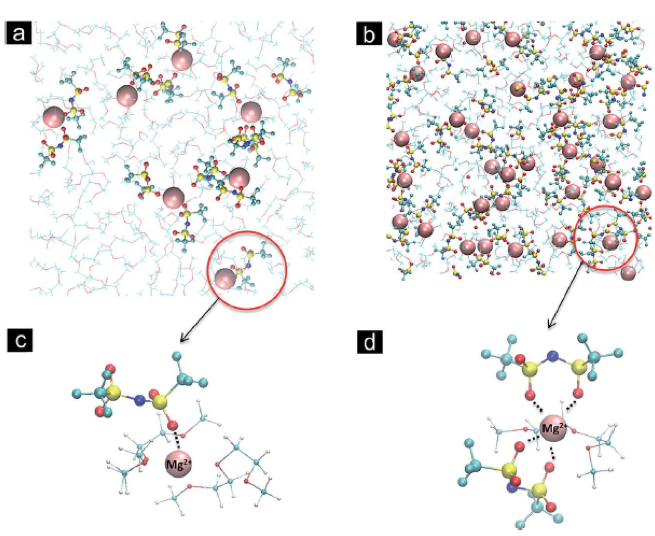
A review of molecular-level interactions governing multivalent electrolyte behavior is presented. The current understanding of solvation structure, stability and transport properties of electrolytes for secondary Mg, Zn and Ca batteries is critically examined from experimental and theoretical studies.
Significance and Impact
Aspects of solvation structure are linked to electrolyte properties such as conductivity, diffusion, and reversible plating/stripping. Future research directions are discussed with an emphasis on rational design.
Research Details
Multivalent electrolyte species exhibiting reversible plating/stripping range from Grignard reagents to 3-D boron clusters, with progressing improvements in electrolyte properties, but fundamental understanding of these working electrolytes is limited.
Multivalent electrolytes are prone to ion-pairing and instability of species in contact with partially reduced cations (e.g. Mg2+ -> Mg+) formed during deposition.
Future improvements to electrolytes are discussed in terms of salt dissociativity, chemical stability during plating/stripping and electrochemical stability window.

