Scientific Achievement
A series of cyclopropenium polymers were synthesized and evaluated as high potential active material for size-exclusion flow batteries.
Significance and Impact
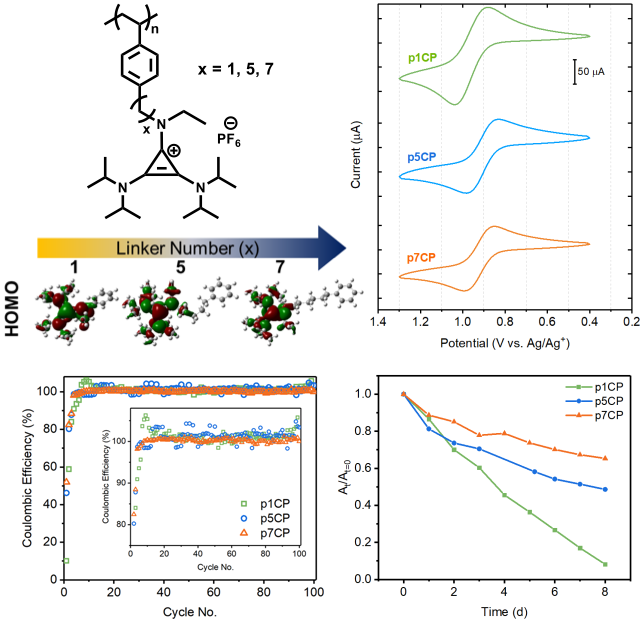
Increasing tether length between the polymer backbone and the charge-storing pendant group improved chemical reversibility and capacity retention, providing insight into materials design for stable redox-active polymers.
Research Details
- Theoretical simulations of varied tether lengths predicted electronic isolation of the cyclopropenium redox group when the backbone was greater than x = 5 methylene groups away.
- Polymers of tethers x = 1, 5, 7 were synthesized and displayed similar redox potentials and low degrees of electrode filming.
- Chemical reversibility and capacity retention of the polymers increased with increasing tethers while cycling at Coulombic efficiencies near 100%.
- Long-term charged state stability also increased with tether length as demonstrated by tracking the absorbance of charged cyclopropenium over an eight-day period.

