(Bottom) A dissolution-redistribution process involving sulfur/polysulfide species at the cathode occurred during the electrochemical process.
Scientific Achievement
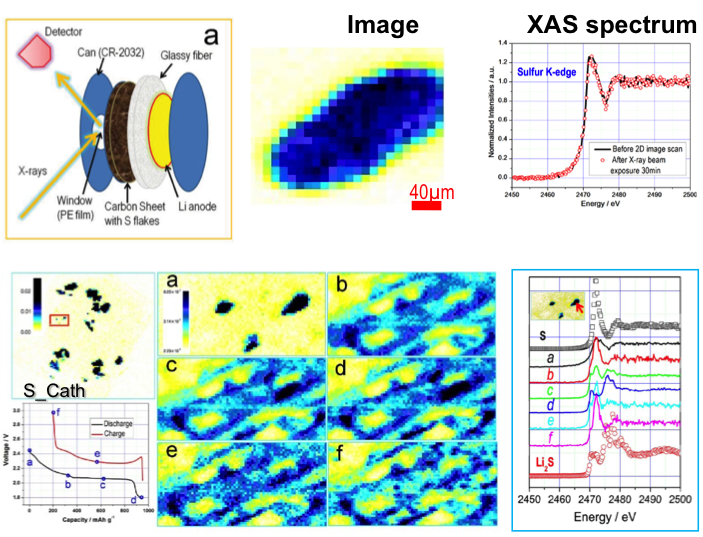
The dissolution and redistribution of sulfur and polysulfides were directly observed by XRF imaging. The long-chain polysulfides were generated as soon as discharge was initiated. The dissolved polysulfide diffused to the anode side and deposited onto (and reacted with) lithium metal.
Significance and Impact
This work provides a powerful tool for investigation of the Li-S system and new insight into the development of Li-S battery technology. Practical design of Li-S batteries must completely integrate the cathode, electrolyte and lithium anode.
Research Details
- In situ XRF microscopy combined with XAS measurements was utilized to track the morphology and chemical state changes of the sulfur electrode in real time throughout an entire first electrochemical cycle.
- Ex-situ XRF microscopy and XAS measurement were performed to detect the redistribution of polysulfides on the lithium anode side of the cell.
Work performed at Brookhaven National Laboratory and Pacific Northwest National Laboratory (JCESR partner) by X. Yu , H. Pan , Y. Zhou , P. Northrup , J. Xiao , S. Bak , M. Liu , K.-W Nam ,D. Qu , J. Liu , Ti. Wu and X.-Q. Yang, Adv. Energy Mater. 2015.

