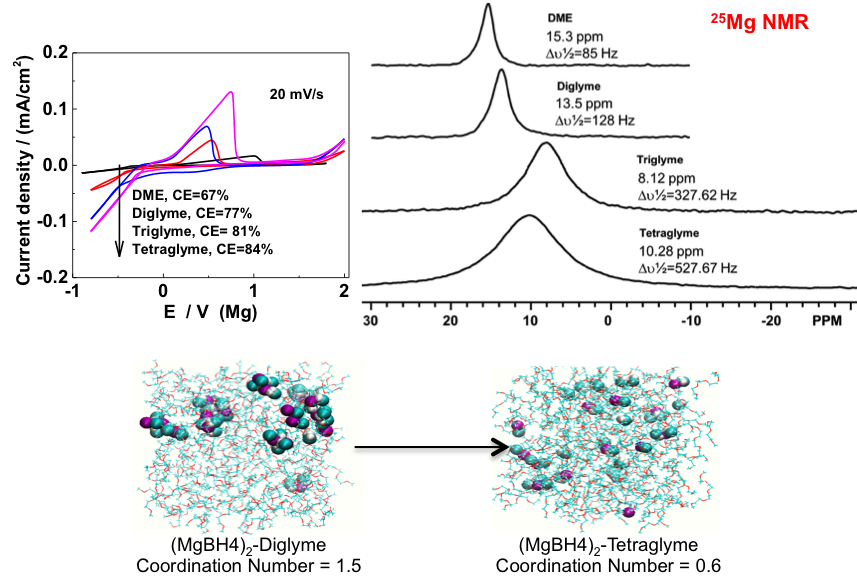
(Right) The chemical shift of 25Mg NMR spectra shift negatively from DME through diglyme to triglyme, which is due to the interaction between Mg and oxygen atoms in glymes through electron donation from oxygen; but the chemical shift moves back for tetraglyme, which is explained as the high dissociation of Mg(BH4)2 in tetraglyme leading to free BH4-.
(Bottom) Snapshot from MD simulations for Mg(BH4)2-diglyme (left) and Mg(BH4)2-tetraglyme (right) showing the average coordination numbers of the Mg cation with the anion in the electrolyte.
Scientific Achievement
- Multivalent salts are prone to ion-pair formation in many solvents, particularly the glymes that provide stability at metal anode
- Integration of modeling and NMR techniques has led to an understanding of the glyme ligand effect on the electrochemical properties of Mg electrolytes
- Design metrics including solvent oxygen chain length and chelating properties are identified as promising avenues toward improved solvation of the active cation
Significance and Impact
- Increasing glyme chain length has increased the solvation of the Mg2+ cation, reducing the ion-pair formation, and improved the electrochemical properties of the electrolyte at the metal anode
- The ligand effect will be used to further electrolyte design in other systems
Research Details
The electrochemical properties of Mg(BH4)2 is enhanced with stronger solvent interaction with cation, which promotes Mg(BH4)2 dissociation.
Work performed at Pacific Northwest National Laboratory (JCESR Partner) Yuyan Shao, Jianzhi Hu, Tianbiao Liu, Mark Engelhard and Jun Liu, Lawrence Berkeley National Laboratory (JCESR Partner), Nav Nidhi Rajput and Kristin Persson

