(Bottom) Results of an X-ray spectroscopy (EDX) line scan, acquired horizontally through the center of two “diamond” unit cells. This confirms the presence of Mg in the tetrahedral sites, and the expected atomic arrangement of Mn-O-Mg-Mg-O-Mn in the MgMn2O4 structure.
Scientific Achievement
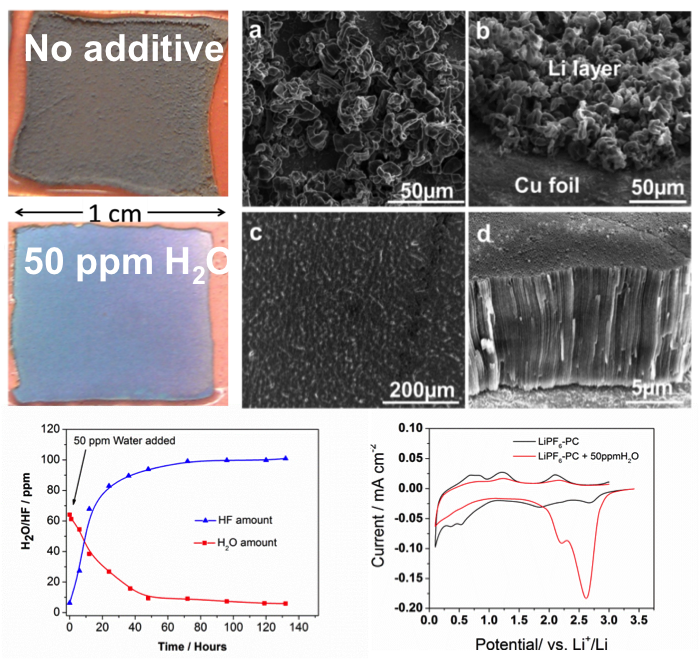
First demonstration of reversible insertion of multivalent magnesium ions (Mg2+) into a spinel-type manganese oxide (Mn2O4), using multi-modal characterization
Significance and Impact
- Proof of Mg2+ insertion provides an avenue to designing a high voltage cathode for a magnesium-based battery that surpasses current lithium-ion technology
- Breakthrough enabled by JCESR Sprint establishing strategic collaborations on specific fundamental issues
Research Details
- Reversible Mg intercalation into Mn2O4 observed in both aqueous and non-aqueous electrolytes. Results from four different tools provided insight at different scales.
- Reaction between Mn2O4 and MgMn2O4 observed at an average voltage of 2.9 V vs. Mg2+/Mg0. Theoretical specific energy: 783 Wh/kg (compared to 400 Wh/kg in today’s lithium-ion).
Work performed at the University of Illinois at Chicago and SLAC National Accelerator Laboratory (JCESR partners) and Argonne National Laboratory (JCESR managing partner) by C Kim, PJ Phillips, B Key, T Yi, D Nordlund, Y-S Yu, RD Bayliss, S-D Han, M He, AK Burrell, RF Klie and J Cabana, Advanced Materials

