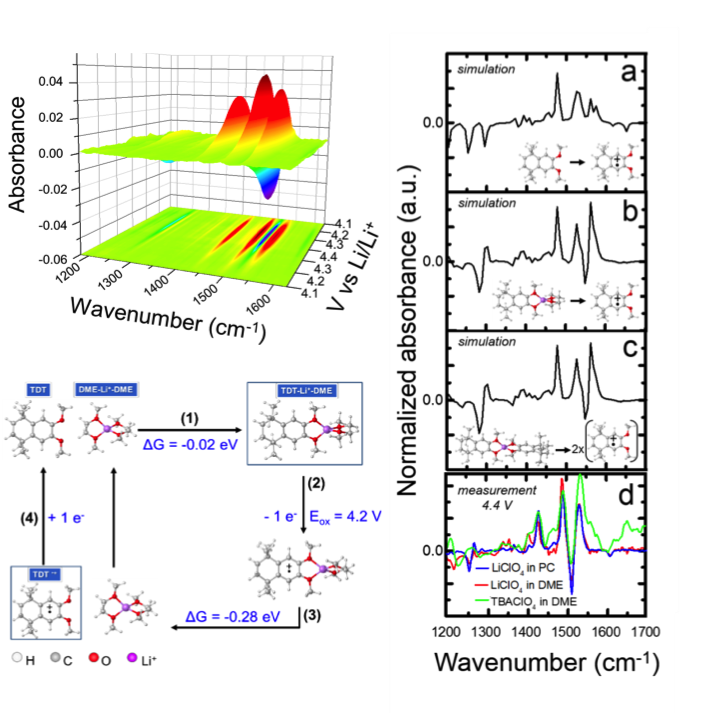
Scientific Achievement
Exhaustive DFT experiments are well-matched to in-situ spectroscopic data showing Li+ coordination to basic methoxy groups. Li+ coordination promotes improved redox reversibility within Li+ electrolytes.
Significance and Impact
Redox-mediated Li+ interactions are shown in spectroscopic data. Enhancement of stability via interactions with the electrolyte can be considered as part of the design strategy.
Research Details
- Electrochemical characterization by voltammetry and bulk electrolysis revealed kinetics, stability are sensitive toward electrolyte selection.
- Structural interactions during cycling in Li+ and non-Li+ electrolyte were probed by in situ FTIR at the EDL.
- DFT was used to predict reaction pathways and guide interpretation of the spectroscopic data.
TDT was provided by Argonne’s Materials Engineering Research Facility (MERF) under the name ANL-RS21. Work was performed at Massachusetts Institute of Technology (JCESR collaborator) and Argonne National Laboratory (JCESR managing partner) by Carino, Emily; Staszak-Jirkovsky, Jakub; Assary, Rajeev; Curtiss, Larry; Markovic, Nenad; Brushett, Fikile R., Chem. Mater. 2016.

