Scientific Achievement
A computationally inexpensive model has been developed to parametrically study the cyclic voltammetry behavior of magnesium-based battery electrolytes.
Significance and Impact
The model enables rapid parameterization of the kinetics and transport properties of novel electrode/electrolyte systems, even with only a limited amount of experimental input. This will allow for examination of the differences between these systems and determination of how parameters may need to be optimized for superior performance.
Research Details
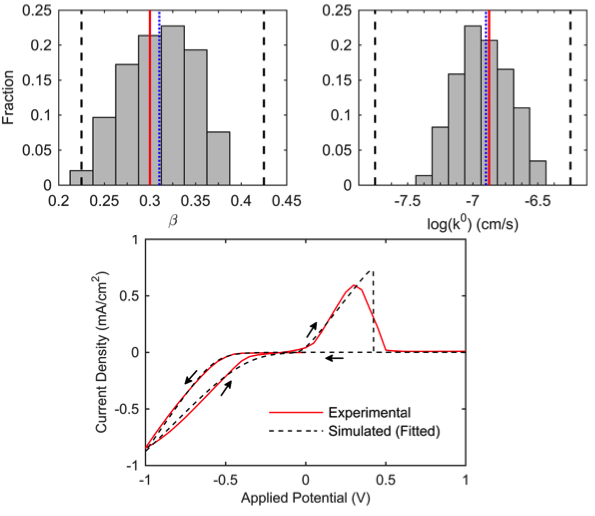
- A numerical model was developed from non-electroneutral dilute solution theory that considers magnesium reaction kinetics; nucleation, growth, and stripping of magnesium deposits; and the Coulombic efficiency of the electrode/electrolyte system.
- Using a batch-fitting process, likely ranges of the governing parameters were determined by comparing the simulated voltammogram to experimentally obtained data for a Mg(BH4)2-based electrolyte.
Work performed at University of Michigan (JCESR partner)
and University of Oxford by A.F. Chadwick, G. Vardar, S. DeWitt, A.E.S Sleightholme, C.W. Monroe, D.J. Siegel, and K. Thornton. J. Electrochem. Soc., 2016, 9, A1813-A1821

