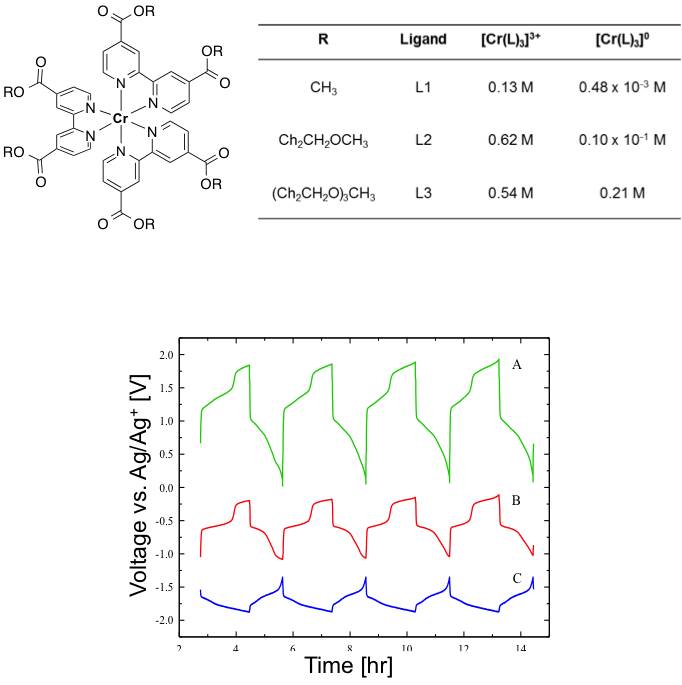
(Bottom) Charge-discharge curves for the Cr(L3)3 complex: A) Full H-cell potential, B) Positive electrode potential, and C) Negative electrode potential. Multiple plateaus are observed, indicating multi-electron transfer.
Scientific Achievement
Designed and synthesized functionalized chromium-bipyridine complexes with high solubilities that undergo up to 6 electron transfers over a ~2 V window
Significance and Impact
- Significantly enhanced solubility by functionalizing non-innocent ligand scaffold
- Demonstrated symmetric cell that achieved multiple electron transfer at positive and negative electrodes
- Developed strategies to manipulate solubilities and electrochemistries of metal coordination complexes that could be used to enhance other active species
Research Details
- Characterized variations in solubility as a function of state of charge for bipyridine complexes
- Potentials of the positive and negative charge/discharge plateaus are consistent with the redox couples observed in cyclic voltammetry
Work was performed at the University of Michigan (JCESR Partner), Department of Chemistry and Department of Chemical Engineering by P. J. Cabrera, X. Yang, J. A. Suttil, K. L. Hawthorne, R. E. M. Brooner, M. S. Sanford, and L. T. Thompson, Journal of Physical Chemistry C, 2015.

