Research Highlights
-
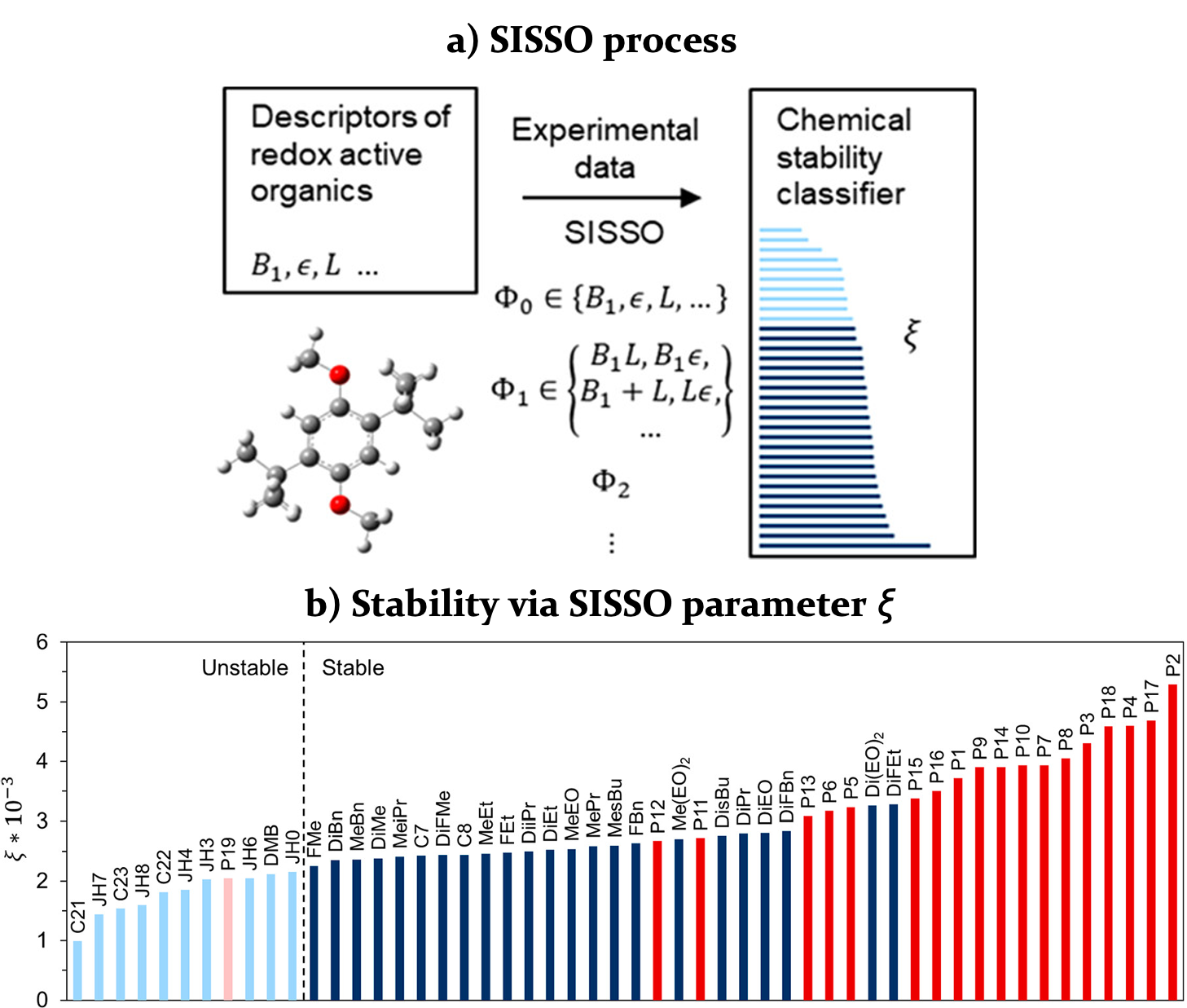
Cross-Platform Classifier for Electrochemical Stability
Predicting electrochemical stability of redox-active molecules is needed to achieve durable flow batteries for electric grid storage. Here, Sure Independence Screening and Satisfying Operator (SISSO) is used to construct a binary classifier for predicting chemical stability of charged organic molecules. SISSO is an artificial intelligence (AI) expert that derives and tests heuristic algebraic formulas to rationalize data trends. Two unrelated families of redox-active molecules were classified by the same empirical parameter, which is a nonlinear combination of molecular descriptors found by SISSO without human guidance. Read More
-
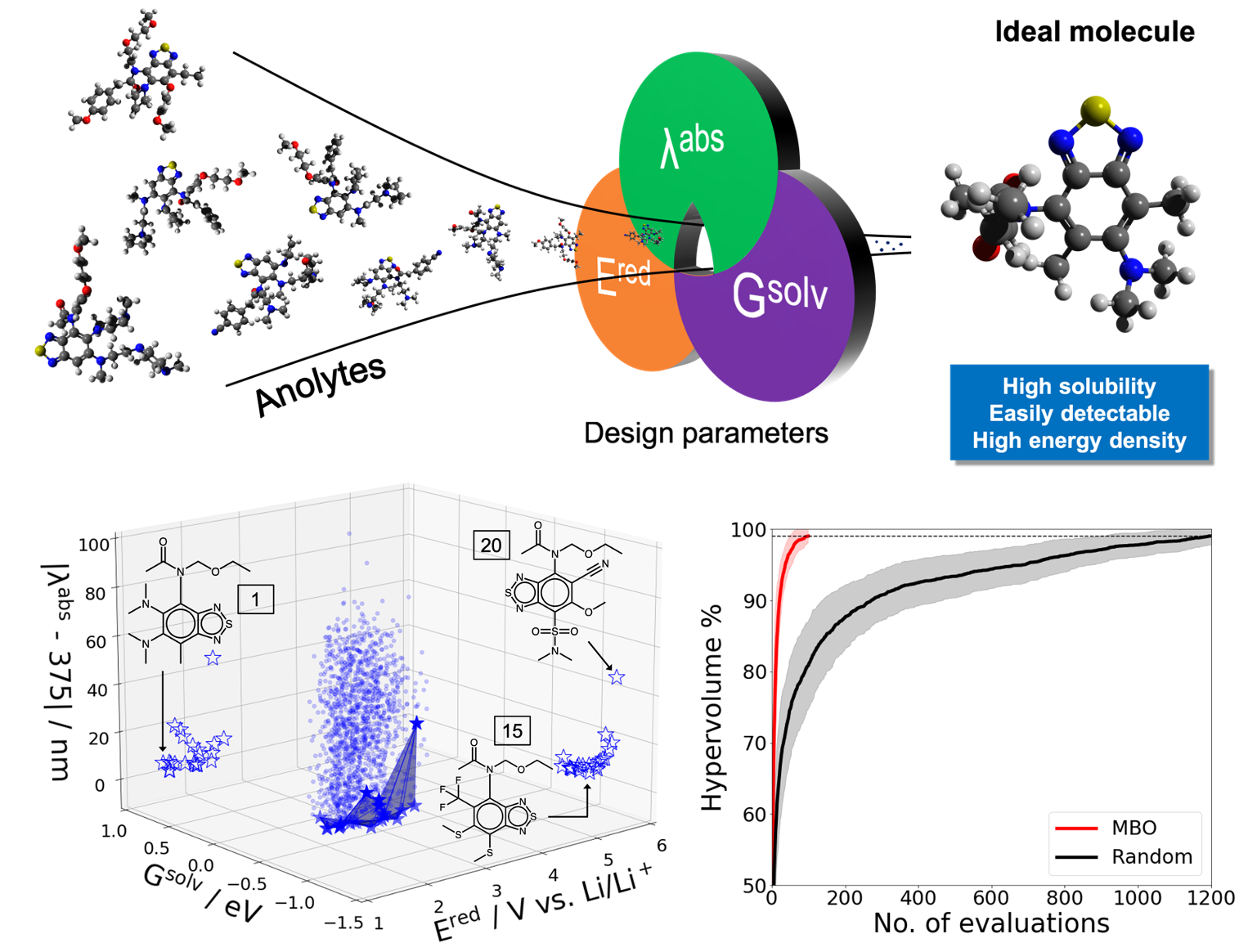
Discovery of Energy Storage Materials Using Multi-objective Bayesian Optimization
Designing optimal materials for redox flow batteries is challenging due to the multi-criteria requirement and large number of potential candidates. Multi-objective Bayesian optimization (MBO) was developed and demonstrated to significantly accelerate (15-fold) the identification of anolyte molecules of desired reduction potential, solubility, and fluorescence from a large chemical space. Read More
-
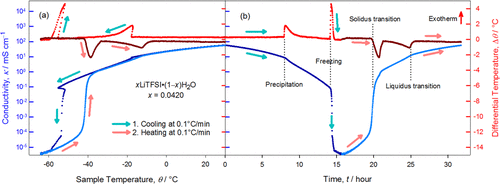
Understanding the transient ion transport behavior at phase transition temperatures
In this work we clarified a fundamental question of high importance but often neglected: how the ion transport behavior of electrolytes change during phase transition. The quantitative understanding of such transient behavior has profound impact on how to accurately define and predict electrolyte applications in extreme environments. Read More
-
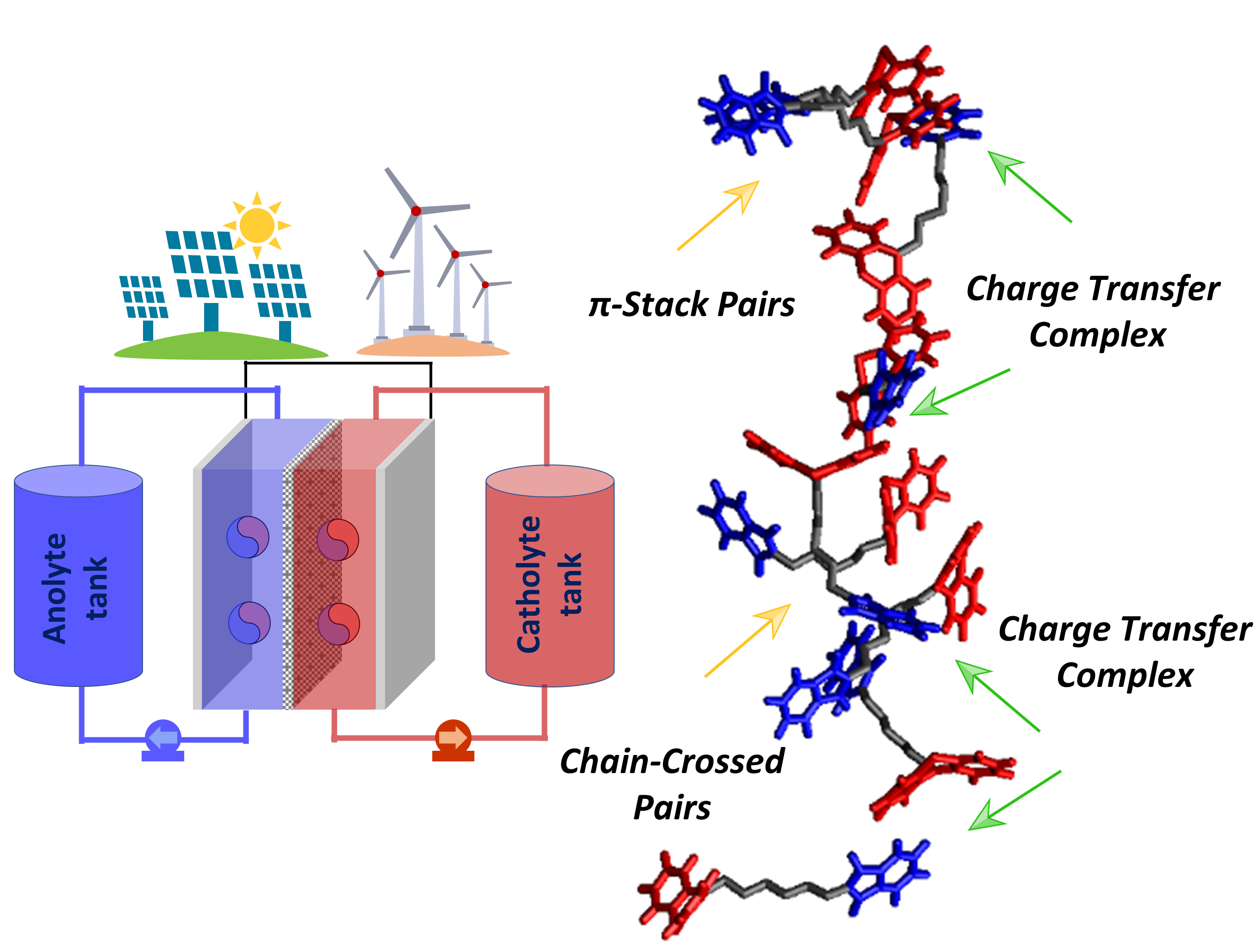
Critical Role of Structural Order in Bipolar Redox‐Active Molecules for Organic Redox Flow Batteries
Bipolar redox-active molecules have been suggested as a means to address crossover related issues in all-organic redox flow batteries. The present work describes a fundamental rubric to uncover the likely origins in the performance metrics of these bipolar molecules that are salient for their use in redox flow batteries. Read More
-
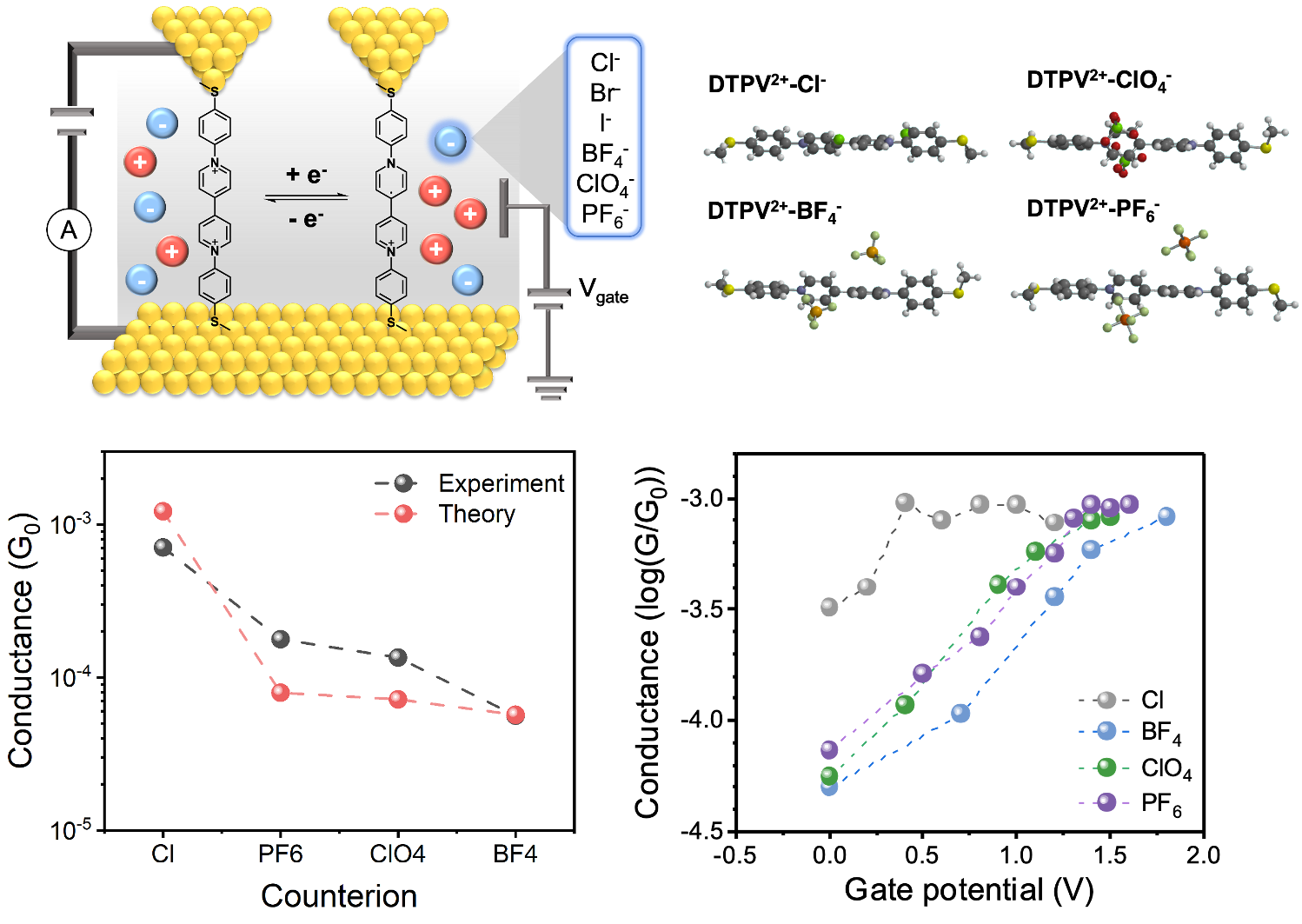
Counterion-dependent charge transport in viologens
Our results show that charge transport in viologens critically relies the electrochemical environment and is particularly sensitive to the electrolyte counteranion. We use an electrochemical gating technique to understand charge transport in viologens as a function of the redox state and electrochemical environment. Molecular modeling shows that differences in charge transport arise from counterion-induced variations in molecular geometries. Read More
-
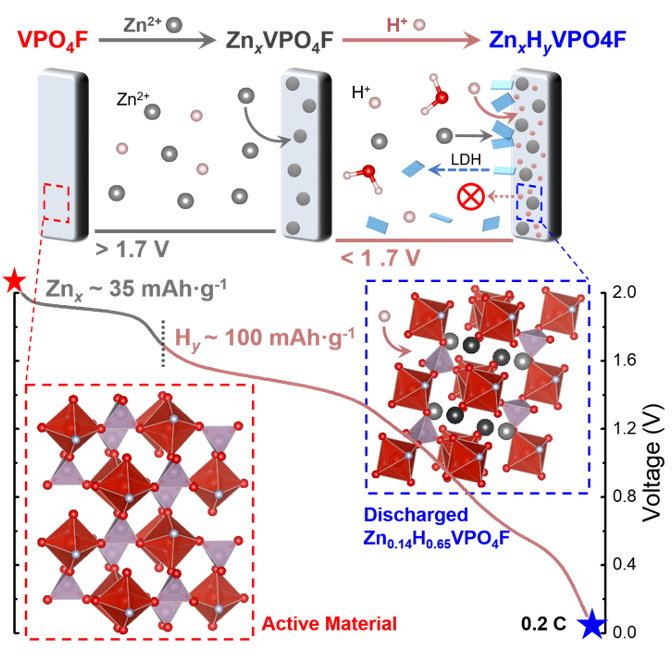
Quantifying and Suppressing Proton Intercalation to Enable High-Voltage Zn-Ion Batteries
The relative contributions of Zn2+ and H+ insertion to the overall capacity of VPO4F are differentiated and quantified in aqueous and hybrid electrolytes. Increasing the salt concentration and/or proportion of nonaqueous solvent successfully suppresses water dynamics, improving the anodic stability and promoting Zn2+ intercalation over competing processes. Read More
-
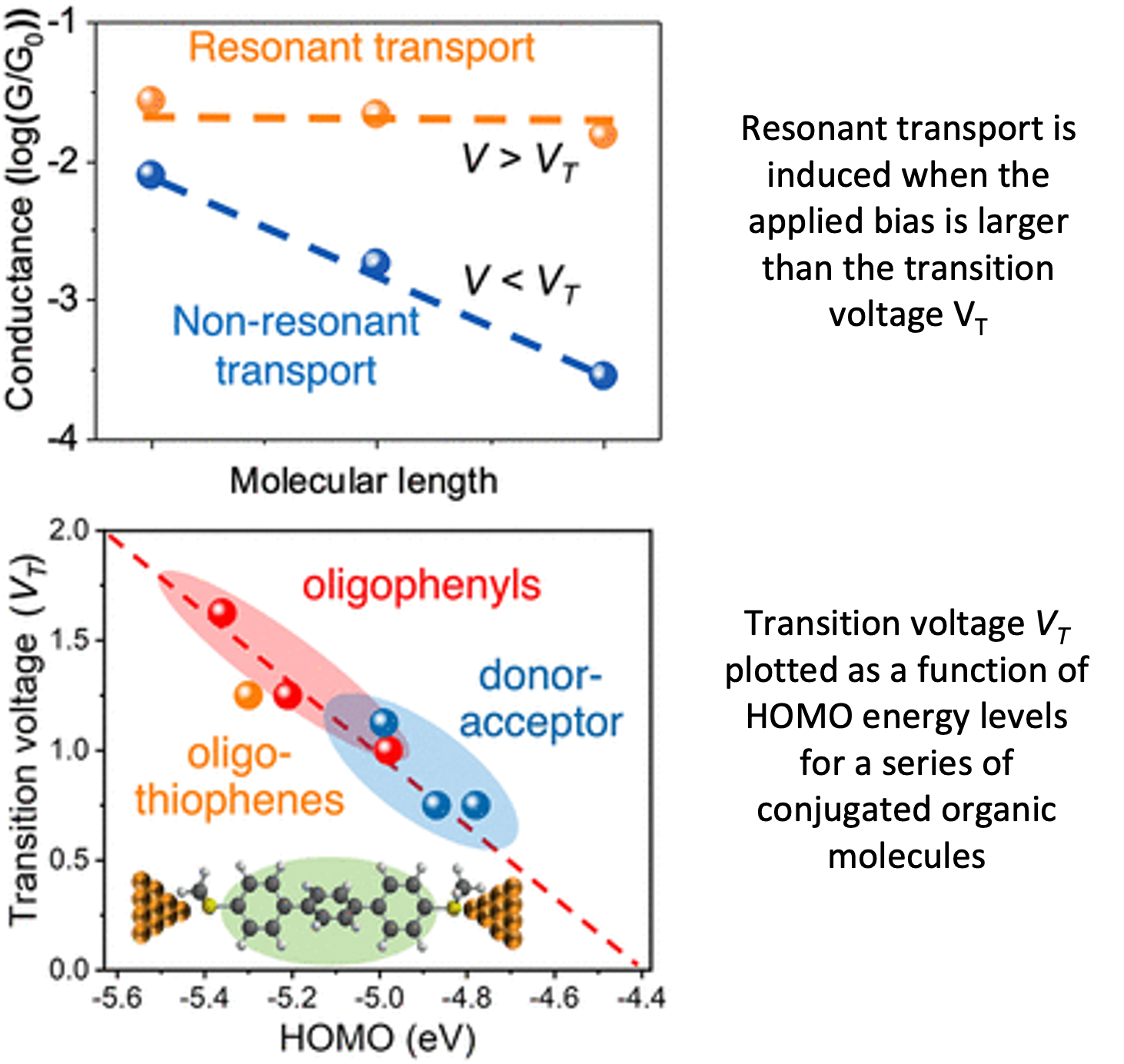
Highly efficient charge transport at molecule-electrode interfaces
Our results show that resonant transport offers a highly efficient intramolecular charge transport mechanism that will enable long-range transport in electronic devices. We further develop a general quantitative relation to describe the non-resonant to resonant transition voltage VT as a function of molecular properties. Read More
-
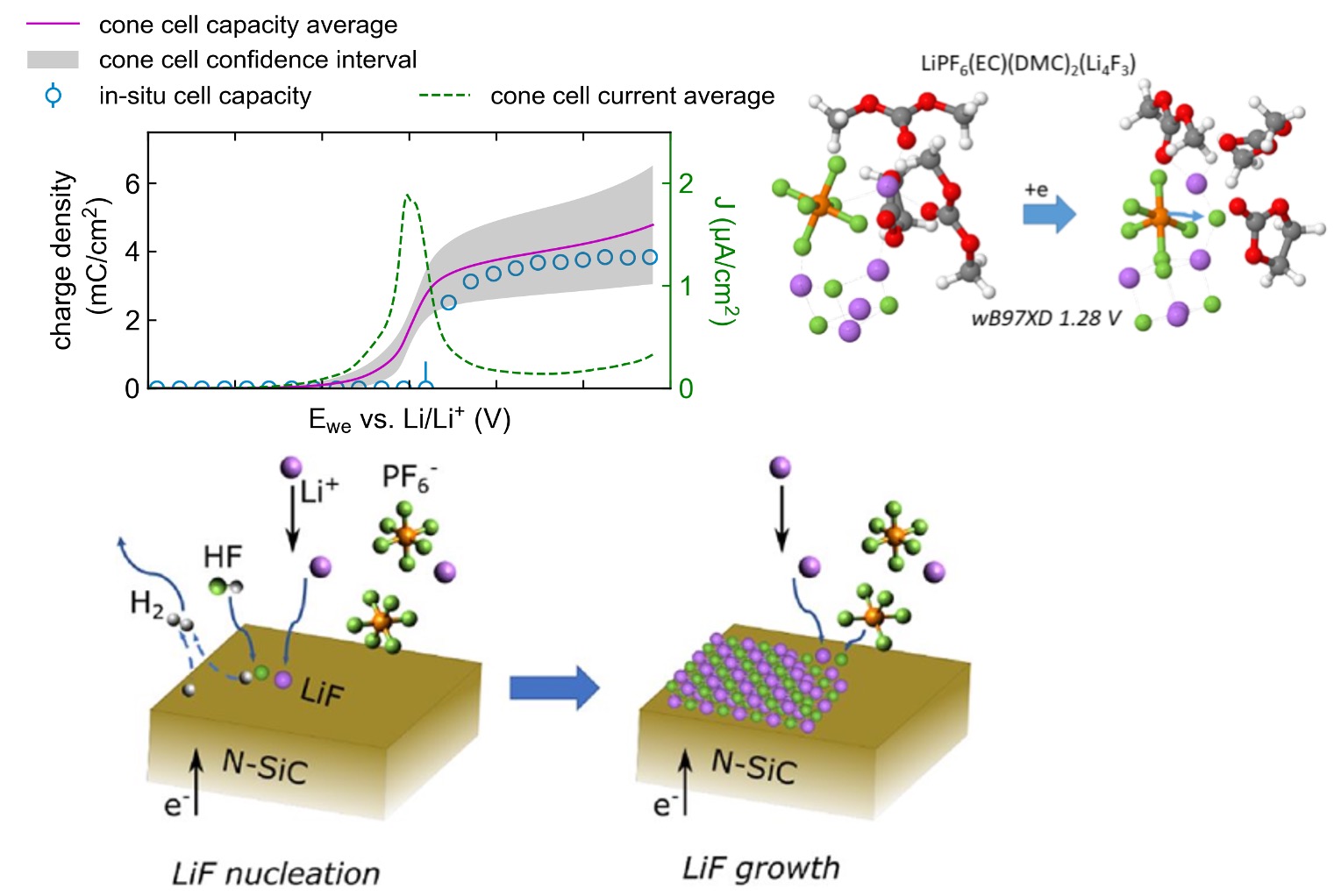
Toward Unraveling the Origin of Lithium Fluoride in the Solid Electrolyte Interphase
We revealed the origin of LiF in the solid electrolyte interphase (SEI) using a multimodal experimental and theoretical approach and found that LiF nucleated via the electrocatalytic transformation of HF followed by significant direct anion reduction. Read More
-
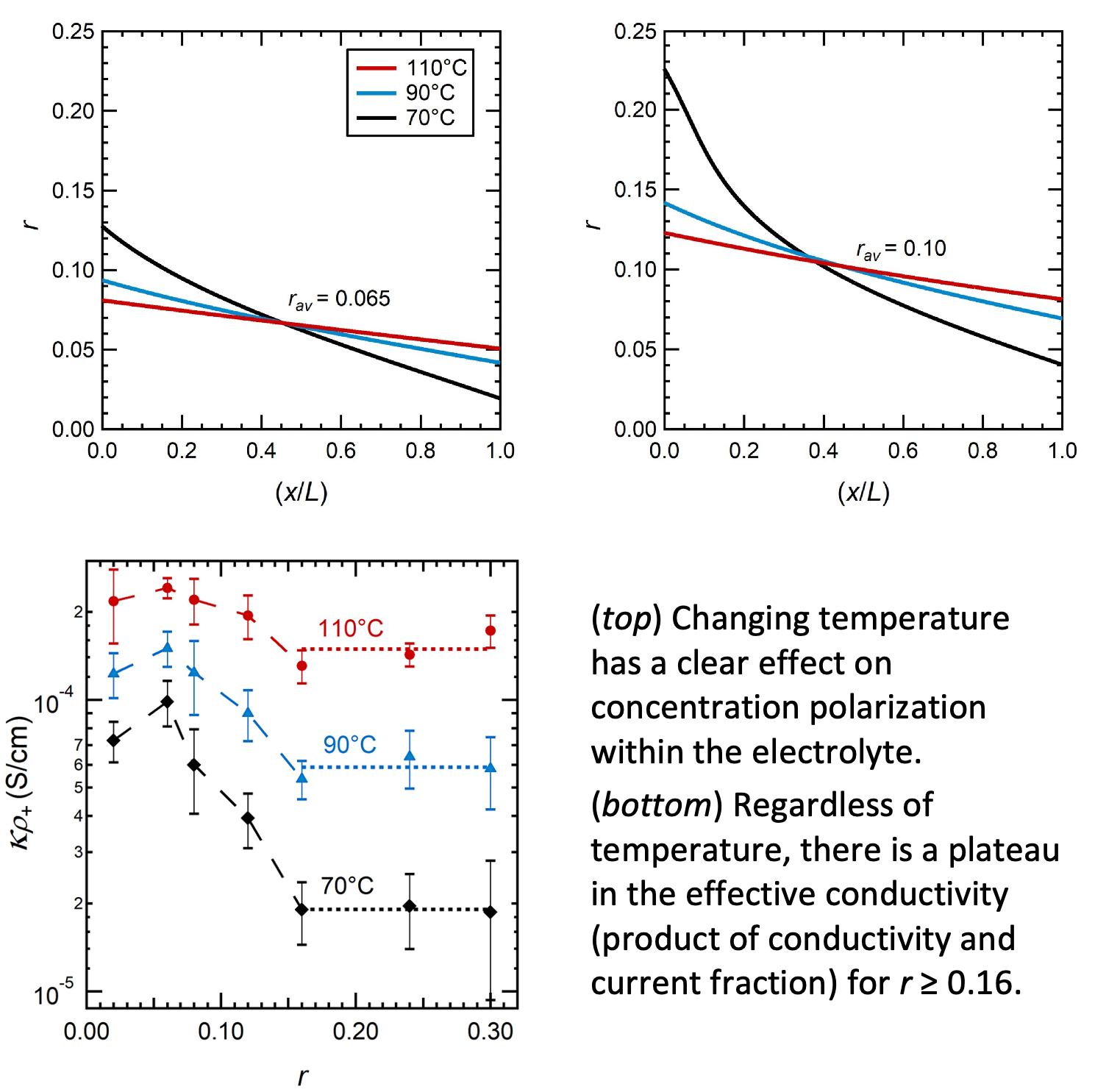
Temperature Dependence of Ion Transport Properties in PEO Electrolytes
We measured the temperature dependence of ion transport properties in PEO/LITFSI electrolytes across a range of salt concentrations. Using these results, we were able to predict how concentration polarization changes with temperature. Read More
-
Water or Anion? Zn2+ Solvation Environment in Mixed Zn2+/Li+ Water-in-Salt Electrolytes
Zn2+ cations are mainly solvated by six waters in their first solvation shell, while TFSI- anions are excluded from solvation in the WISE electrolyte consisting of 1 m Zn(TFSI)2 and 20 m LiTFSI although the TFSI- concentration is as high as 22 m. Read More
Latest Updates
-
You’re Invited - JCESR and Beyond: Translating the Basic Science of Batteries
Please join us at Argonne National Laboratory on Tuesday, April 4, 2023 for JCESR and Beyond: Translating the Basic Science of Batteries. Registration is now open. This in-person event will celebrate 10 years of research from the Joint Center… Read More
-
A Message from JCESR: In Memory of George Crabtree
It is with heavy hearts that we say goodbye to George Crabtree, a Senior Scientist and Distinguished Fellow at Argonne National Laboratory, and Director of the Joint Center for Energy Storage Research (JCESR), who passed away unexpectedly on January 23. Dr. Read More
-
Cyanopyridines As Extremely Low-Reduction-Potential Anolytes for Nonaqueous Redox Flow Batteries
Discovery of a cyanophenylpyridine derivative with a very low reduction potential and good stability during cycling. Read More
-
Characterizing Redoxmer – Electrode Kinetics Using a SECM-Based Spot Analysis Method
Identified asymmetries in electron transfer (ET) kinetics between the reduction and oxidation of ferrocene-based redoxmers by measuring the ET rate constants (kf/kb) as a function of electrode potential. Read More
-
Benzotriazoles as Low Potential Anolytes for Non-Aqueous Redox Flow Batteries
We developed an easy-to-synthesize benzotriazole-based anolyte with a high energy redox potential (-2.3 V vs Fc/Fc+) and high solubility that demonstrates stable electrochemical cycling performance. Read More

