Scientific Achievement
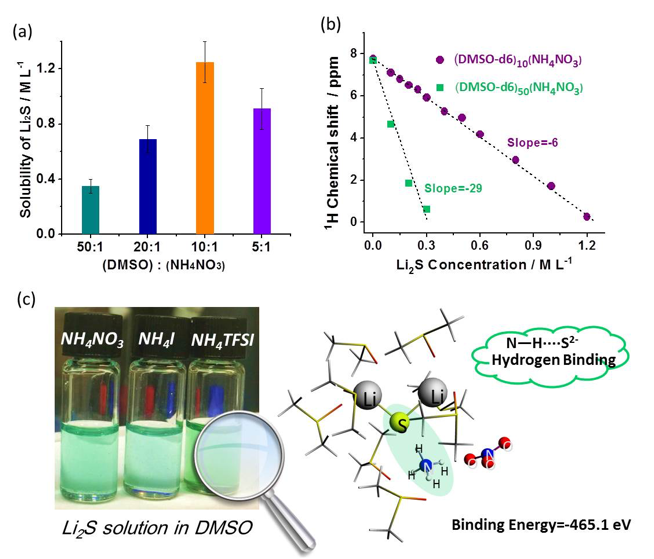
Ammonium salts are demonstrated as effective additives to promote the dissolution of Li2S (up to concentrations of 1.25 M) in DMSO solvent at room temperature through hydrogen binding between N-H groups and S2– anions.
Significance and Impact
Dissolving Li2S in an organic electrolyte is a facile solution to maintain the active reaction interface between an electrolyte and a sulfur cathode, and thus addresses the issue of uncontrollable passivation of electrodes by highly insulating Li2S that limits sulfur utilization, increases polarization, and decreases cycling stability in Li-S batteries.
Research Details
- Different ammonium salts were identified as effective additives for dissolving Li2S as well as other short chain polysulfides.
- NMR measurements and DFT simulations reveal strong interactions between NH4+ and S2– via hydrogen bonding (N―H····S2-) that facilitates the dissolution of Li2S.

