Scientific Achievement
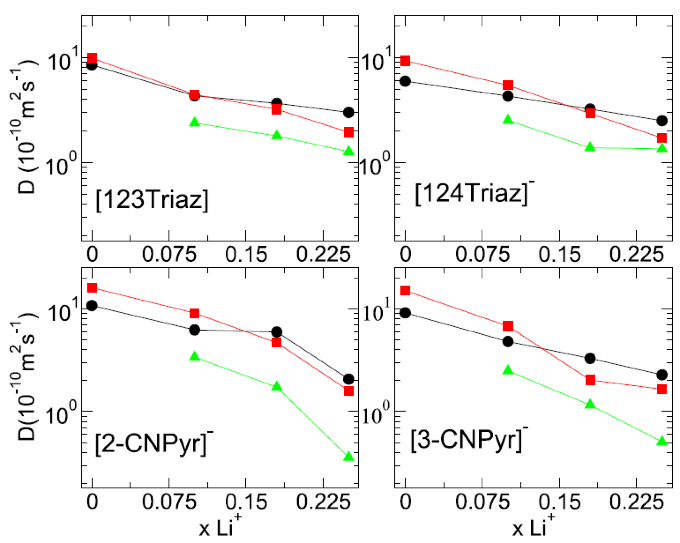
Molecular dynamics simulation was carried out on 12 aprotic heterocyclic anion ionic liquid based electrolyte systems and (methyloxymethyl)triethylphosphonium triazolide was found to have the best performance.
Significance and Impact
Self-diffusivities, lithium transference numbers and free volumes were computed for each system and fundamental understanding of the performance was provided.
Research Details
- It was found that the lithium solvation environment depends just on the anion structures, whereas IL cation has negligible influence.
- The addition of lithium to the ionic liquids lowers the free volume, which correlates with the reduction in dynamics.

