Scientific Achievement
Demonstrated that alkali metal can be used as a catalyst Li-O2 cell cathode design and opens the possibility of future optimization of functional K-doping in carbon cathode materials
Significance and Impact
Provides essential scientific understanding for optimizing the energy and reversibility of the Li-O2 system discharge and charge chemistry, which potentially has 10 times the energy density of Li-ion batteries
Research Details
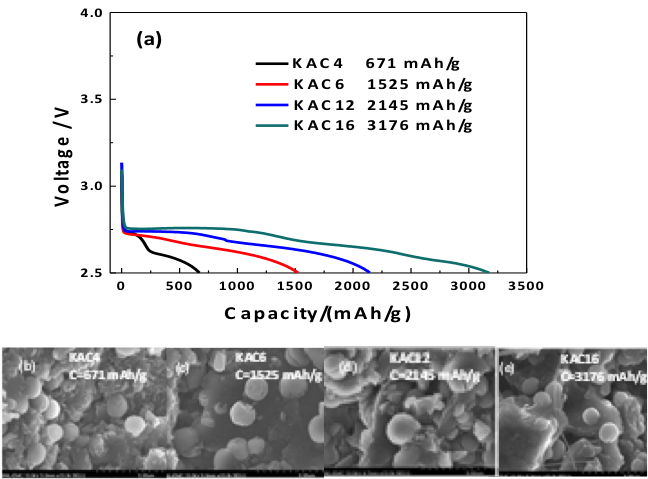
- Small amounts of K impurity (0.05 to 0.43 weight%) deliberately introduced into activated carbon cathodes by synthesis are found to have a dramatic effect on Li-O2 cell performance
- Increasing the amount of K in the cathode was found to increase the capacity (700 to 3200 mAh/g), cycle life (1 to 12 cycles), and round trip efficiency (66 to 75%) of Li-O2 cell
- DFT studies suggest that K is an effective oxygen reduction catalyst that can be intercalated in graphitic layers and at defect sites
Work performed at Argonne National Laboratory (JCESR managing partner) and Sandia National Laboratories (JCESR partner) by D. Zhai, K.C. Lau, H-H Wang, J. Wen, D.J. Miller, F. Kang, B. Li, K. Zavadil, and L.A. Curtiss, Effect of Potassium Impurities Deliberately Introduced into Cathode Materials on the Electrochemical Performance of a Li-O2 Battery, ChemSusChem., 2015.

